To receive more expert insights on the latest healthcare news, connect with us.
ICER’s Use of FDA Approval Volume to Calculate Budget Impact Thresholds: A Scenario Analysis
Summary
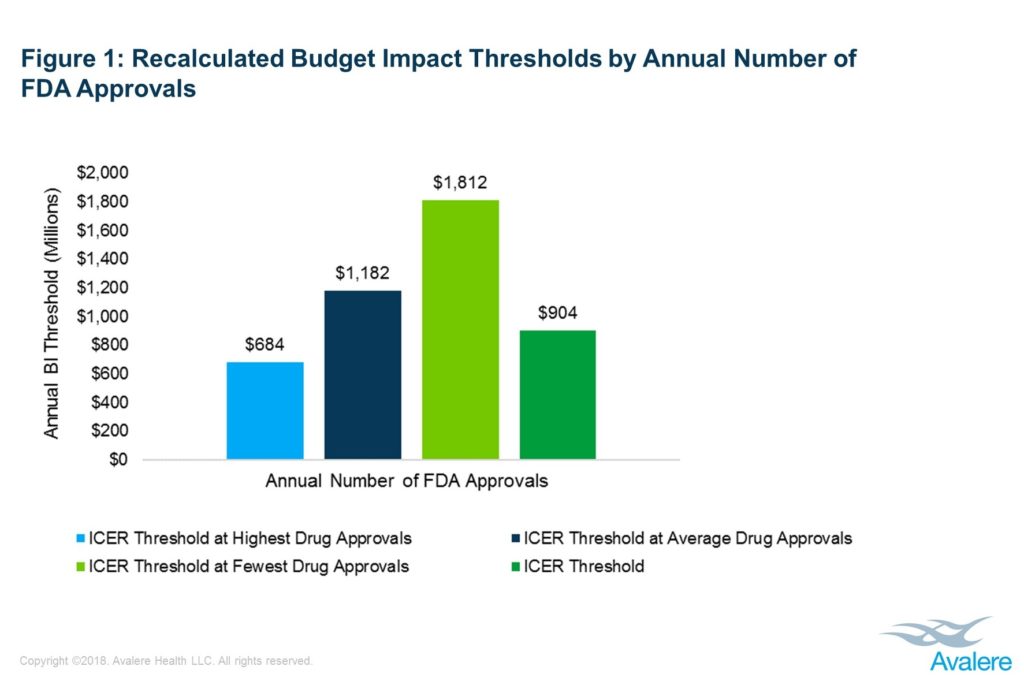
ICER’s reliance on the average number of FDA drug approvals to calculate budget impact thresholds leads to significant variability.As part of its value framework, the Institute for Clinical and Economic Review (ICER) conducts a budget impact analysis to estimate the short-term affordability of a product or service in relation to an internally-developed cost threshold. Over the last 3 years, ICER has revised its budget impact threshold from $904 million for 2015-2016 to $915 million for 2017-2018 to a current value $991 million.
One of the primary inputs that ICER uses to calculate its budget impact threshold is the number of new drugs approved by the U.S. Food and Drug Administration (FDA) per year, a metric that can vary year to year. In this report, Avalere examines the impact of using new drug approval volume on ICER’s budget impact thresholds.
Avalere’s analysis finds that because the number of FDA drug approvals can vary dramatically, ICER’s reliance on the number of FDA drug approvals can lead to substantial and unpredictable variability in the budget impact threshold, which is important when used as a benchmark for affordability. For example, utilizing the lowest, average, and highest yearly number of FDA approvals during a 20-year period results in budget impact thresholds of $1.81 billion, $1.18 billion, and $684 million, respectively, in comparison to ICER’s threshold which has ranged from $904 million to $991 million (Figure 1).
Under the ICER methodology, the year in which a product is approved determines the budget threshold that will be applied to it, and these thresholds vary significantly from year to year. As a result, the year in which a drug is approved plays an important role in determining whether ICER’s “budget alarm” will be triggered and the percentage of patients that ICER estimates can gain access before restrictions would need to be applied. This wide and arbitrary variation in ICER’s budget threshold may limit its reliability as a benchmark. Stakeholders relying on ICER’s conclusions may wish to appropriately contextualize their interpretations to ensure that decisions impacting patient access to medications result from methodologically sound approaches.
Download the report for a full discussion of our research findings and methodology.
Funding for this research was provided by The Pharmaceutical Research and Manufacturers of America (PhRMA). Avalere maintained full editorial control.
January 23, 11 AM ET
Learn More