A large life sciences manufacturer anticipated that some of its products may be selected for drug price negotiations under the Inflation Reduction Act (IRA). It sought to create an evidence strategy to articulate the value of its products during negotiations and thus needed to predict how CMS might weigh evidence and evaluate results. We helped the client anticipate CMS’s approach to evaluating evidence packages for products during the negotiation process, and develop strategies to strengthen those packages, to ensure that negotiated maximum fair prices (MFPs) accurately reflect the value of the client’s products.
Featured Insight
Trusted Partnership
We partner closely with clients to ensure their IRA strategy is tailored to their industry position, needs, and goals, helping clients make informed decisions and achieve long-term success.
Insight
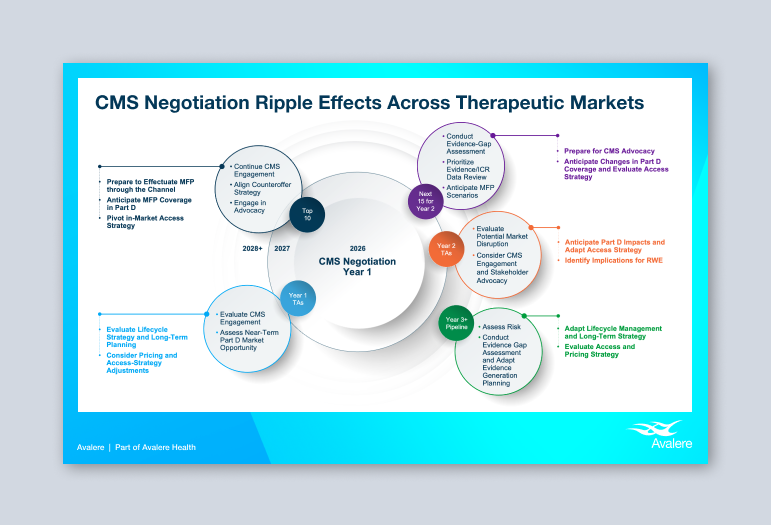
Planning for Life Sciences
Medicare drug price negotiation will have long-lasting ripple effects across therapeutic markets, and manufacturers will need to adopt a product-specific approach to stay ahead of the changing drug landscape. Avalere experts in policy, CMS engagement, market access, and evidence assessment help manufacturers reassess their strategies to stay ahead of the changing drug landscape.
Engage an Avalere Expert Today


Navigating the Medicare Prescription Payment Plan: What It Means for Part D Plan Sponsors
The MPPP introduces new considerations for Part D plans’ financial risk, operations, and enrollee engagement.

Navigating the Medicare Prescription Payment Plan: What It Means for Manufacturers
The MPPP will help improve Part D patient affordability, prompting manufacturers to reassess existing patient assistance programs and educational efforts.

IRA Inflation Rebate Impact on Part B Beneficiary OOP Costs
Avalere finds that between 0.1% and 0.2% of Medicare FFS beneficiaries would experience lower OOP costs for the Part B drugs subject to Q3 2024 inflation rebates.

IRA Negotiations May Affect Millions of Medicare Beneficiaries
Maximum fair prices for the first 10 selected drugs may shift therapeutic dynamics and have direct and indirect impact to millions of beneficiaries.

Commercial Spillover Impact of Part B Negotiations on Physicians
Physicians could lose at least $25 billion in add-on payments for 10 Part B drugs expected to be negotiated by CMS, with oncology products accounting for at least $12 billion.

Navigating the Medicare Prescription Payment Plan: What It Means for Stakeholders
The Medicare Prescription Payment Plan will change how Medicare beneficiaries manage their Part D out-of-pocket costs, with implications across stakeholders.

Key Considerations for MFP Effectuation and the 340B Rebate Model
Stakeholders consider implementation of a 340B rebate model to address duplicate discount risk.

Manufacturer Learnings from the First Round of Negotiated MFPs
Avalere experts share initial impressions of the publicly released negotiated MFPs for the first round of selected drugs, highlighting implications to industry.
Potential Impacts of IPAY 2026 Maximum Fair Prices on Health Plan Formulary Negotiations
With the release of the IPAY 2026 MFPs, health plans should analyze the impact to formularies and identify opportunities for contracting changes.

Health Policy in the 2024 Election: Avalere and Outside Experts Weigh In
With the election fast approaching, former HHS Secretary Alex Azar, Dan Mendelson, and Avalere experts discussed implications for healthcare stakeholders.
 Please wait ...
Please wait ...


