COVID-19 Therapies Could Benefit from NTAP Reimbursement Opportunity
Summary
Therapies currently being developed to treat COVID-19 in the inpatient setting have opportunity for additional Medicare reimbursement.In response to the COVID-19 pandemic, drug manufacturers are responding in rapid fashion, developing or repurposing drug therapies to target the virus directly or to address the cascade of side effects that may result in pulmonary respiratory failure.
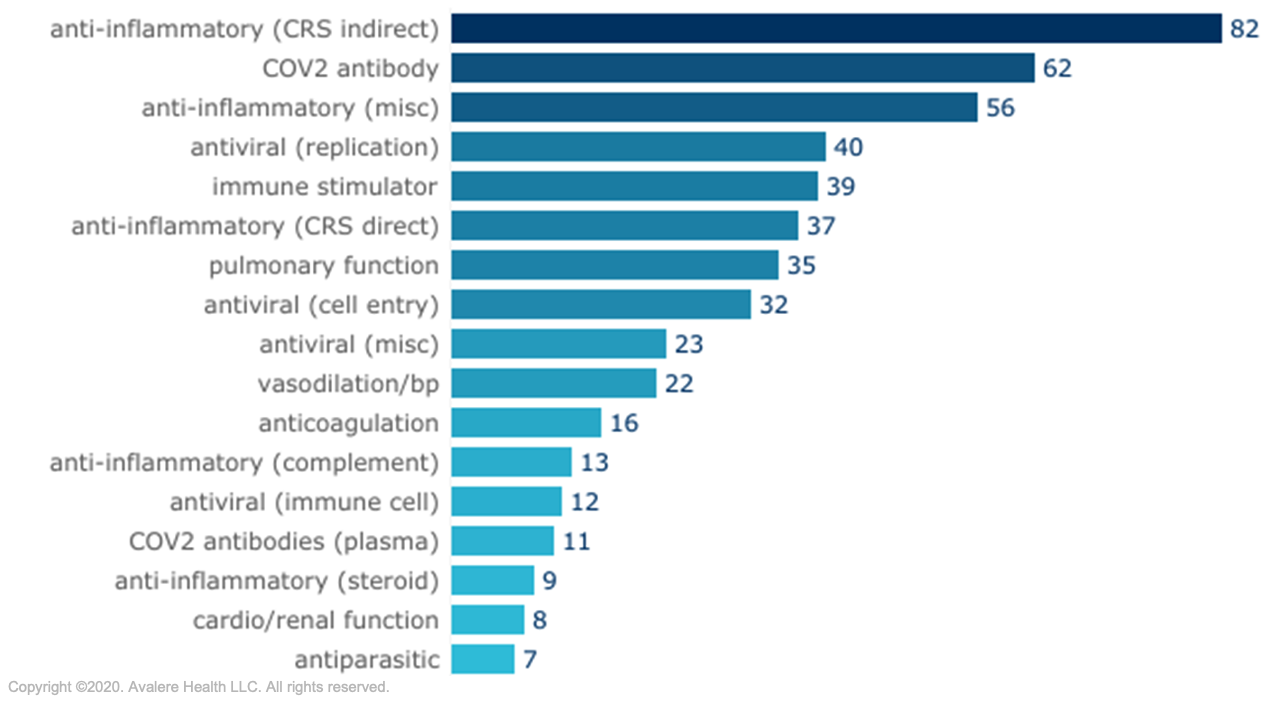
Source: BIO COVID-19 Therapeutic Development Tracker, accessed August 30, 2020.
An important consideration for manufacturers is the cost impact to hospital systems using these treatments. A significant portion of the severely ill, hospitalized patients afflicted with COVID-19 are elderly people covered by Medicare.
The Medicare program pays hospitals 1 bundled payment covering all costs incurred for providing services during the inpatient stay, including drugs, devices, and supplies. In certain cases, however, Medicare provides a separate, additional payment above the standard payment amount under the New Technology Add-On Payment (NTAP) policy that has been in place since 2003. To be eligible for NTAP, a technology must meet 3 criteria: newness, cost, and clinical improvement.
COVID-19 therapies are likely to meet all 3 NTAP criteria, which could result in hospitals receiving additional payments when using those therapies to treat Medicare patients, equaling either 65% of the estimated cost of the drug or 75% if the drug has been approved by the Food and Drug Administration (FDA) under the Qualified Infectious Disease Product status. NTAP can improve overall hospital economics as it reduces the cost burden associated with adopting and utilizing a new technology which will be critical to appropriately and successfully treat COVID-19 patients.
Manufacturers of COVID-19 therapies need to act now to ensure consideration for NTAP beginning in fiscal year (FY) 2022 (i.e., October 1, 2021). Applications for next year’s cycle will be due in early October 2020. If that deadline is missed, manufacturers will have to wait another year to seek NTAP approval. Additionally, products seeking NTAP do not need the FDA’s approval upon submission of the NTAP application in October but must have approval prior to the next FY. For more information on NTAP requirements and submission timeline, see our previous insight, “NTAP Eligibility Criteria Opportunities for Inpatient Reimbursement.”
| NTAP Activity | Traditional Pathway | Alternative Pathway | Alternative Pathway with Proposed Conditional Approval |
|---|---|---|---|
| Submission Deadline for NTAP Application | Oct 2020 | Oct 2020 | Oct 2020 |
| Presentation at New Technology Town Hall | Dec 2020 | * | * |
| Submission of ICD-10-PCS Code Request | Dec 2020 | Dec 2020 | Dec 2020 |
| Submission of Additional NTAP Application Information | Dec 2020 | Dec 2020 | Dec 2020 |
| Presentation at ICD-10 C&M Committee Meeting | Mar 2021 | Mar 2021 | Mar 2021 |
| Release of IPPS Proposed Rule | May 2021 | May 2021 | May 2021 |
| Deadline for FDA Approval | Jul 1, 2021 | Jul 1, 2021 | Prior to Jul 1, 2022 |
| Release IPPS Final Rule | Aug 2021 | Aug 2021 | Aug 2021 |
| If Awarded, NTAP Effective | Oct 1, 2021 | Oct 1, 2021 | Quarter after FDA approval |
*The New Technology Town Hall’s purpose is to present information regarding the clinical improvement criteria. As alternative pathway applicants only have to meet the cost criteria, the applicant may choose not to attend.</P
Certain drugs designated by the FDA as a qualified infectious disease product (QIDP) or devices with FDA breakthrough status may apply for NTAP using an alternative pathway to NTAP. Additionally, the CMS proposed in FY 2021 IPPS rule to allow products approved through the FDA’s Limited Population Pathway for Antibacterial and Antifungal Drugs (LPAD) to use the alternative pathway. The CMS also proposed to allow QIDP and LPAD products to have conditional approval, which means once FDA approval is received, the NTAP would begin at the start of the next quarter as opposed to the following fiscal year. All technologies allowed to use the alternative pathway only need to satisfy the cost criterion to qualify for NTAP designation. All other products must follow the traditional pathway and satisfy all three NTAP criteria (newness, cost, and significant clinical improvement).
Avalere is advising life science companies, healthcare providers, and stakeholders across the healthcare industry to improve access to new therapies. To learn more about our work, connect with us.
January 23, 11 AM ET
Learn More



