Comparing Medicare FFS Pneumococcal Vaccination Rates in 2019 and 2020
Summary
A new study from Avalere analyzes age, race and ethnicity, and Medicare-Medicaid dual-eligibility status of Medicare Fee-For-Service (FFS) beneficiaries with pneumococcal vaccine claims in 2019 and 2020.Background
Adults in the US are recommended to receive routine immunizations against 13 vaccine preventable diseases (VPDs), including COVID-19. However, immunization uptake rates vary across the adult population, with the Medicare population consistently lagging Healthy People 2020 targets. A previous Avalere analysis found that between 2016 and 2018, care associated with VPDs cost Medicare an estimated $106.4 billion.
As stakeholders consider strategies to increase adult vaccine access and uptake, understanding utilization trends across the broader population and within specific sub-populations and high-risk groups is important. Findings can help identify where disparities may exist and inform potential approaches targeting increased and equitable access across the entire population.
To that end, Avalere conducted a claims-based analysis examining Medicare FFS pneumococcal polysaccharide vaccine (PPSV23) claims from 2019 and 2020. PPSV23 is routinely recommended by the Advisory Committee on Immunization Practices (ACIP) for all adults aged 65 and older, as well as for adults ages 19–64 who fall into defined risk groups with certain chronic or immunocompromising conditions.
Findings
Avalere assessed the number of claims in 2019 and 2020 across 3 cohorts for whom the vaccine is recommended: all adults 65 and older, immunocompromised adults under age 65, and adults with chronic conditions under age 65. For each cohort, Avalere assessed the percentage change in the vaccination rates from 2019 to 2020. Findings are segmented by age, race and ethnicity, and Medicare-Medicaid dual-eligibility status and provide insight into specific sub-populations that may have been particularly impacted by care disruption during the pandemic.
PPSV23 Vaccination by Age Groups
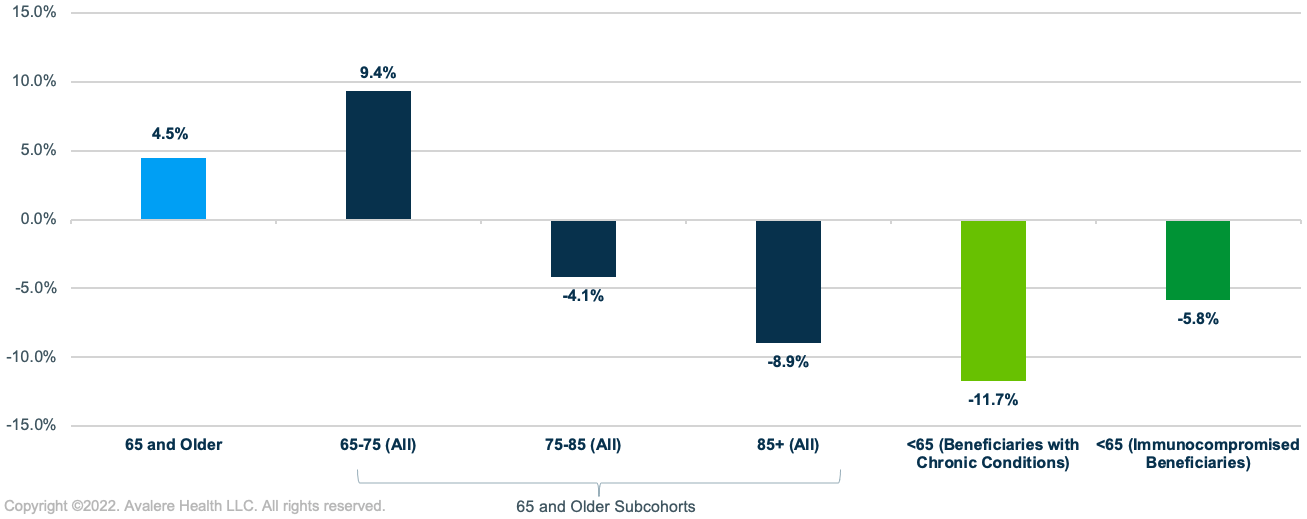
Across Medicare beneficiaries 65 and older, Avalere found a 4.5% increase in PPSV23 vaccination rates between 2019 and 2020.This change was driven primarily by a 9.4% increase in PPSV23 vaccination rates among beneficiaries aged 65–75, the largest group in the 65 and older population (>50%). The percentage change in PPSV23 vaccination rates decreased in older populations, with a 4.1% decrease for adults 75–85 and 8.9% decrease for adults 85 and older. Among adults under 65 in high-risk groups, Avalere found an overall 8.3% decline in PPSV23 vaccination rates from 2019 to 2020, with the largest decrease for beneficiaries with chronic conditions (-11.7%) and a smaller decrease for immunocompromised beneficiaries under 65 (-5.8%) (Figure 1).
PPSV23 Vaccination by Race and Ethnicity
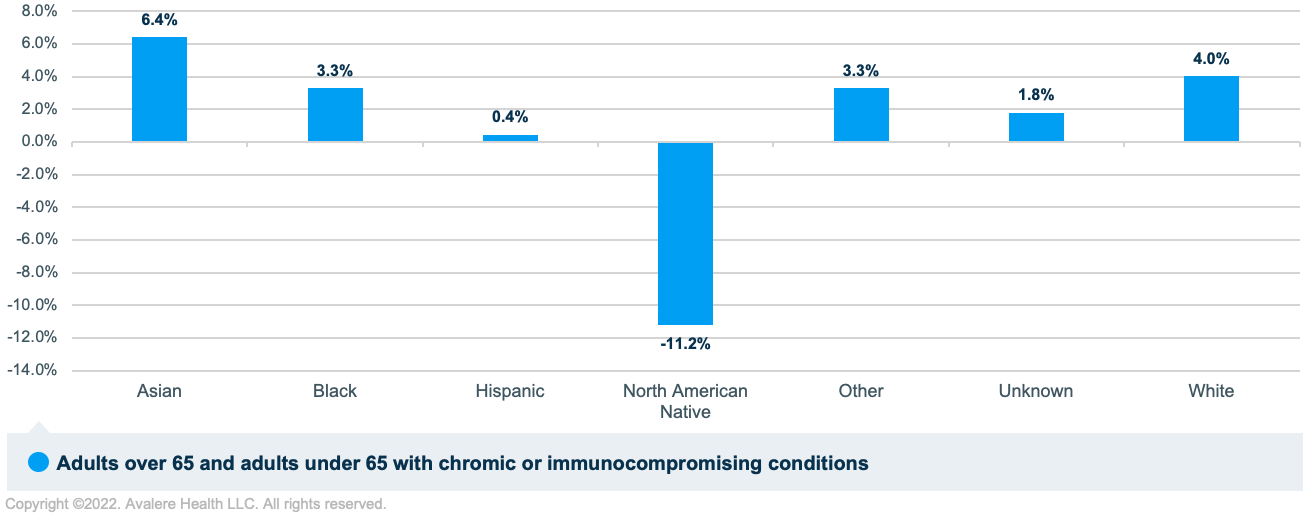
The analysis found differences in the percentage change in 2019 and 2020 PPSV23 vaccination rates when segmented by race and ethnicity. Overall, PPSV23 vaccination rates increased between 2019 and 2020 for all races and ethnicities except the North American Native population, with the largest increases in the Asian (6.4%) and White (4.0%) populations. A large decrease was observed in the vaccination rate for the North American Native population (-11.2%; Figure 2).
PPSV23 Vaccination by Dual-Eligibility Status
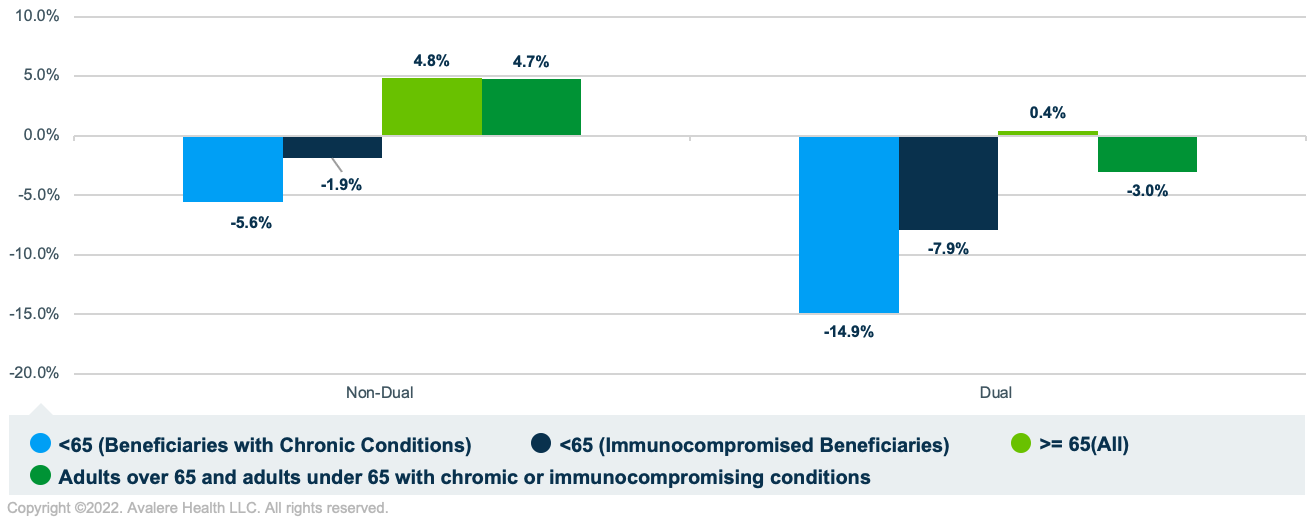
Between 2019 and 2020, PPSV23 vaccination rates decreased for dual-eligible beneficiaries (-3.0%) and increased for non-duals (4.7%). For beneficiaries under 65 with chronic conditions or who were immunocompromised, the PPSV23 vaccination rate decreased more for dual-eligible beneficiaries (-14.9% and -7.9%, respectively) than for non-duals (-5.6% and -1.9%, respectively; Figure 3).
Conclusion
This analysis and similar claims-based analyses conducted by Avalere highlight trends in routine vaccination rates between 2019 and 2020 demonstrating declines among the adult population. These declines in vaccination rates not only underscore the importance of monitoring rates, but also developing programmatic and policy solutions to address immunization rate recovery and further increase rates to levels at and above those demonstrated prior to the pandemic.
Stakeholders such as policy makers and providers may focus on strategies around routine vaccination rate recover and overall uptake for vulnerable populations, such as high-risk groups, beneficiaries aged 75 and older, and dual-eligible enrollees, who experienced greater decreases in PPSV23 vaccination rates between 2019 and 2020 compared to beneficiaries ages 65—75 in the FFS population.
Additionally, understanding differences in uptake among racial and ethnic groups can identify healthcare disparities and inform stakeholder solutions seeking more equitable adult vaccine access across subpopulations. Physicians are a key stakeholder group for initiatives to ensure all recommended patient populations are aware of the pneumococcal vaccine recommendations and offered appropriate vaccinations. Other community-based stakeholders–such as patient and vaccination advocacy groups, public health departments, and community leaders–are also likely partners in advocating for policies and initiatives to support and increase vaccination coverage and confidence.
Data and Methods
To examine PPSV23 claims, this study used 100% Medicare FFS claims (Parts A and B), accessed by Avalere via a research collaboration with Inovalon, Inc. and governed by a research-focused Centers for Medicare and Medicaid Services data use agreement. Data were used for calendar years 2019 and 2020 data and inclusion criteria in given year required at least 6 months of enrollment in Medicare FFS in that year with no enrollment in a Medicare Advantage plan.
Cohorts were created following the ACIP recommendation for PPSV23 vaccination, including in the sample people of any age with chronic conditions, those who are immunocompromised, or with certain underlying medical conditions or other risk factors, as well as anyone 65 years old or older. ICD-10 codes were used to identify beneficiaries with chronic conditions, the immunocompromised, and those with other underlying medical conditions.
Cohorts were examined across a set of demographic characteristics including age, race and ethnicity, rural and urban beneficiaries, and dual eligibility status. Age was based on January of the calendar year, race and ethnicity used Medicare designations, and rural and urban was determined based on beneficiary address. Dual-eligibility status was binary, including partial duals and full duals.
Medicare enrollment and PPSV23 claims in 2019 and 2020 were calculated as intermediary metrics with the key outcomes expressed as the number of PPSV23 claims per 1,000 Medicare beneficiaries in each year.
To learn more about vaccines and preventive services, connect with us.
January 23, 11 AM ET
Learn More



