Study: 57 Percent of Covered Generic Drugs Not on Part D Generic Tiers in 2025
Summary
Over time, the percentage of generic prescription drugs placed on Medicare Part D generic tiers has declined, from 65% in 2016 to 43% in 2025.In Medicare Part D, cost sharing for generic drugs depends on the formulary tiers on which the drugs are placed. Generally, drugs placed on higher formulary tiers have higher cost-sharing requirements than those placed on lower tiers. Generic tiers are typically the first and sometimes second tiers, while the third through fifth tiers are the preferred, non-preferred brand, and specialty tiers, respectively. For plan year (PY) 2025, the average coinsurance is $1.74 for drugs on preferred generic tiers and $6.21 for drugs on generic tiers. Higher tiers have coinsurance ranging from 21% to 40%.
Over the last two years, the Centers for Medicare & Medicaid Services (CMS) has explored ways to lower drug costs for seniors, including a voluntary $2 Medicare Drug List Model, which is intended to limit out-of-pocket costs for a defined list of generic drugs. However, the proposed model does not address the existing formulary design, tiering, and utilization management dynamics that for several years have contributed to higher cost sharing for generics in Part D, and its scope is mainly limited to a small subset of mature generics, which are already covered and preferred by most plans. All 267 drug-dosage combinations on the model list are covered by at least 97% of plans and 255 are on the generic tier of at least 95% of plans.
In November 2024, CMS proposed increasing its review of Part D plan sponsors’ formularies to ensure access to generic drugs and biosimilars. This proposal has the potential to address formulary design issues related to generic and biosimilar tiering and would need to be finalized and implemented by the next administration.
Avalere Analysis
Medicare Part D plans design prescription drug formularies in accordance with CMS requirements. Since 2017, plans have been allowed to include more generic drugs on non-preferred tiers if they replace the “non-preferred brand” tier with a “non-preferred drug” tier. To assess and monitor this policy change, Avalere has routinely analyzed the annual distribution of generic prescription drugs on Medicare Part D tiers (see last year’s analysis).
The most recent update of this analysis examines tier placement from PYs 2016–2025 and finds that Part D plan sponsors continue placing covered generic drugs on non-generic tiers, specifically shifting generic drugs onto the non-preferred tier (Figure 1).
Figure 1. Distribution of Generic Drugs on Generic and Non-Generic Tiers, 2016–2025
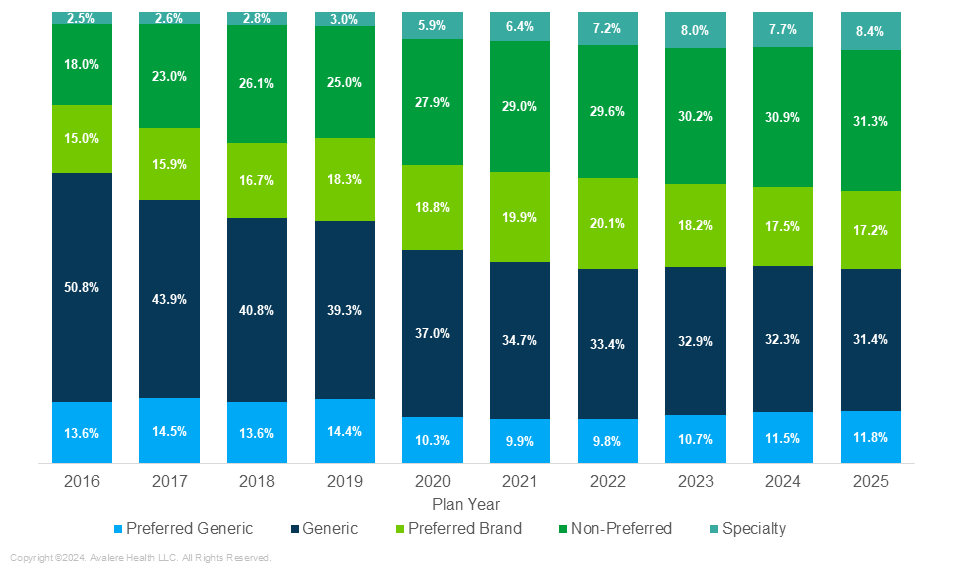
From 2016 to 2025, the percentage of generics placed on the generic tier has decreased by over 20 percentage points. During this same period, the proportion of generic drugs on the non-preferred tier has increase by 13 percentage points. Additionally, the analysis shows growth in the proportion of generics placed on “specialty” tiers, increasing from 2.5% in 2016 to 8.4% in 2025. Smaller changes have been observed in the “preferred generic” tier, which has decreased by 1.8 percentage points, and the “preferred brand,” which has increased by 2.2 percentage points.
Methodology
To conduct the generic tiering analysis, Avalere used the PY 2025 formulary and benefit design information in the Medicare Part D public use files released in October 2024. Avalere assessed the distribution of generic products across five Part D tier categories: preferred generic, generic, preferred brand, non-preferred, and specialty. Tiers were categorized using the Part D tiering data submitted by plans in the Q1 2025 benefits data. Drugs on other or unknown tiers were excluded from these results.
For plans that offer a preferred specialty tier in PY 2025, Avalere grouped the non-preferred and preferred specialty tiers into the specialty tier categorization.
To estimate enrollment-weighted average cost sharing, Avalere used October 2024 enrollment and the 2025 Plan Crosswalk file. Avalere assumes no beneficiaries change plans in 2025.
To calculate coverage and tiering of products on Medicare’s $2 Drug List Model, Avalere leveraged the PY 2025 formulary files and reported results as unweighted plan/drug combination distribution across all products listed on the Medicare $2 Drug List Model Sample Drug List.
Funding for this research was provided by the Association for Accessible Medicines. Avalere Health retained full editorial control.
To receive Avalere updates, connect with us.
January 23, 11 AM ET
Learn More



