2021 State Drug Pricing Legislation: The Evolution Beyond Transparency
Summary
The majority of state legislatures are currently in session, and many states are taking steps to address prescription drug spending and prices through a range of legislative proposals. While states have historically focused on price transparency, state policymakers are now moving beyond those measures to more directly control prescription drug prices through reference pricing, affordability review boards setting upper payment limits, and other price control mechanisms.Prior State Legislative Activity
Twenty-two states have enacted drug pricing laws since 2016. Many of these laws aim to increase drug price transparency through reporting and notification requirements, such as by requiring manufacturers to provide specific pricing information to the state or other entities (e.g., health plans).
Notably, some state transparency laws have been subject to litigation. For instance, in 2018, the 4th US Circuit Court of Appeals ruled that Maryland’s drug pricing law passed in 2017, which was the first of its kind, was unconstitutional. The law would have prohibited a manufacturer or wholesale distributor from engaging in “price gouging,” defined as an “unconscionable increase” in the price of a prescription drug in the original bill, in the sale of an essential generic drug. The appeals court found the law to be unconstitutional because it “directly regulates the price of transactions that occur outside Maryland.” Maryland has continued to pursue transparency efforts, enacting the first-in-nation drug affordability review board in 2019.
Current Legislative Activity
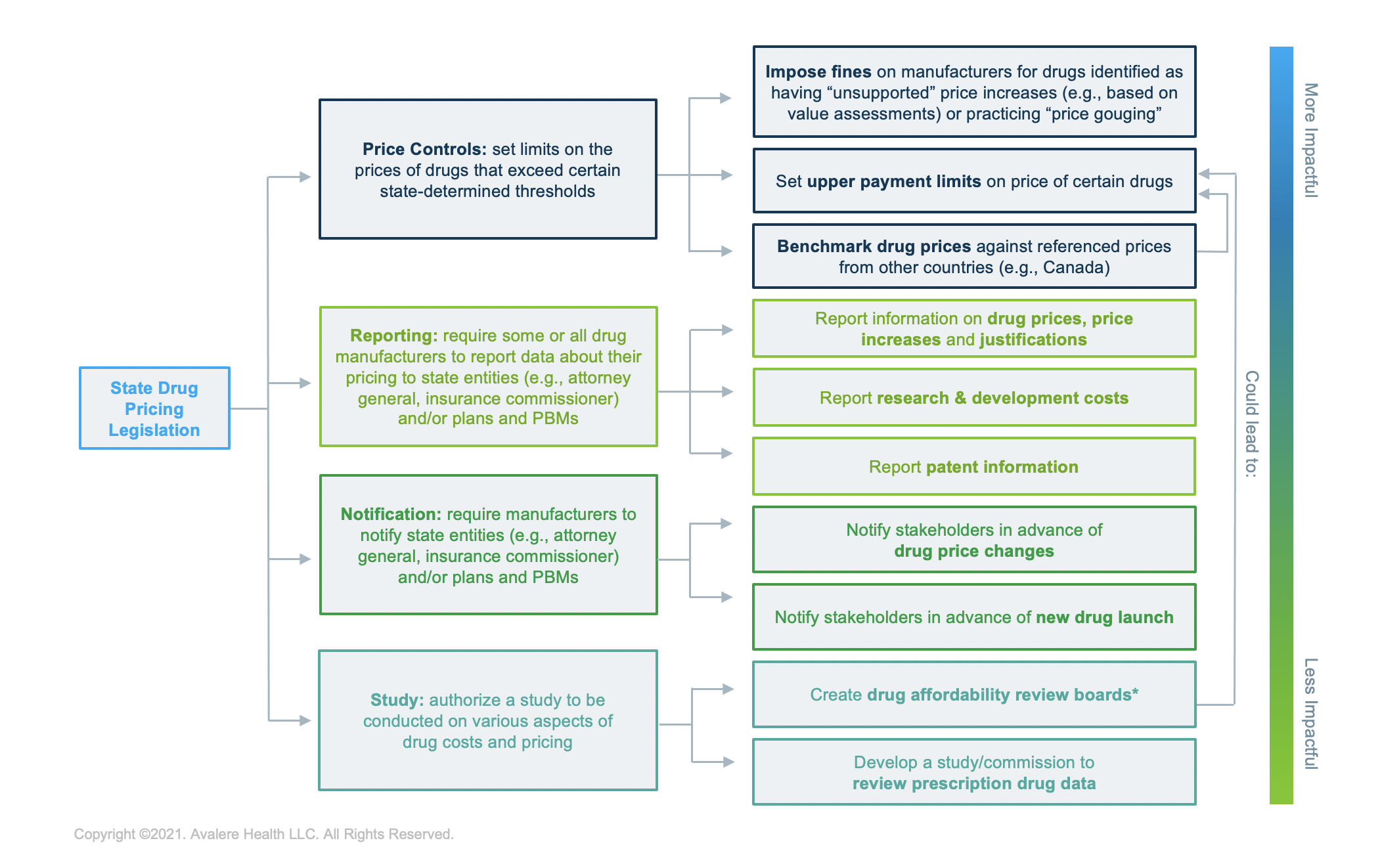
While states continue to consider price transparency initiatives, state legislatures have debated in 2021 new types of drug pricing bills that move beyond manufacturer reporting requirements and more towards directly controlling or limiting prices. In particular, at least 14 states are considering a form of “price control” legislation so far in 2021. The provisions in these bills include, but are not limited to:
- Imposing fines on manufacturers that have “unsupported price increases”—defined by states as increasing drug prices beyond a set inflation rate or beyond an increase threshold determined through value assessments; or
- Benchmarking drug prices in the state against referenced prices from other countries (e.g., Canada)
Similarly, states are evolving the role of drug price affordability review boards to have a more active role in price setting by including new provisions, such as:
- Establishing an upper payment limit1 that could enable a review board to fine manufacturers that exceed the limit set by the review board
- Requiring manufacturers to pay an annual assessment fee to the review board
Recent prescription drug price transparency and cost-focused legislation fall into the following categories, including through a combination of related drug pricing initiatives:
Looking Ahead
Both Republicans and Democrats have signaled that drug pricing is a priority at the state and federal levels. Federal proposals have been mirrored across multiple state legislatures this year (e.g., reference pricing and value-based pricing assessments). Given the substantial state legislative activity so far in 2021, it is likely additional states will enact new laws related to drug pricing and transparency this year. At the same time, states continue to grapple with addressing healthcare costs and increased state budget deficits amidst the COVID-19 pandemic, which could factor into the likelihood of these and other health-related proposals advancing.
To learn more about how we track and analyze emerging state issues, check out State Policy 360TM. Stay ahead of emerging state policy activity. Monitor what’s important for your business across all 50 states and DC, and see major trends in Medicaid waivers, drug benefit management, prescription drug pricing, and individual market changes.
For more on drug pricing trends and news, connect with us.
Notes
- Not in reference to Upper Payment Limits in Medicaid.
January 23, 11 AM ET
Learn More



