Cell and Gene Therapy Pipeline Patient Affordability Opportunities
Summary
With a number of cell and gene therapies (CGTs) expected to obtain Food & Drug Administration (FDA) approval in the next 3 years, stakeholders must consider the unique patient access and affordability barriers that may limit patient ability to receive these novel treatments. Stakeholders should explore affordability solutions that can optimize access, or they risk excluding patients from obtaining potentially life-saving treatments.To date, FDA-approved CGTs have primarily aimed to address the healthcare needs of relatively small patient populations. Though these therapies have largely demonstrated clinical effectiveness and durability, the costs associated with these therapies may prove challenging for patients seeking treatment. Pre- and post-treatment costs associated with patients’ treatment journeys can include diagnostic tests, provider visits, travel, and follow-up monitoring—each with their own cost or cost-sharing requirement. Collectively, those costs can create additional barriers to access and affordability for patients seeking these innovative treatments.
As these therapies continue to enter the market, stakeholders such as patients, caregivers, providers, payers, and manufacturers must consider the total cost-of-care burden for patients and develop mitigation strategies to help address these potential hurdles where possible. Multiple alternative reimbursement and financing solutions can address potential access barriers for CGTs, but opportunities also exist to implement patient-focused affordability solutions to help alleviate patient financial burdens and address the unique needs that these patients face.
A Complex Care Journey
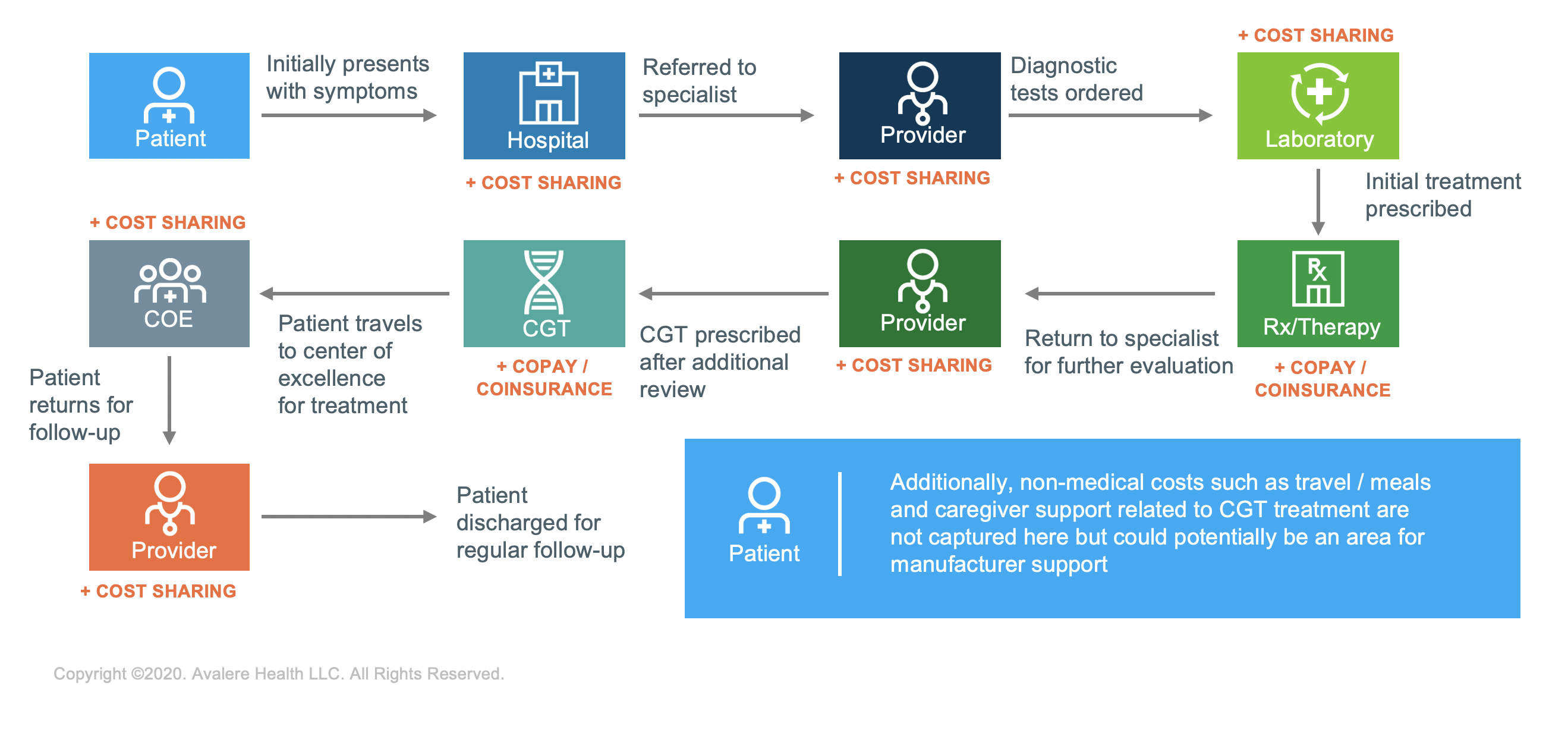
Patients prescribed CGTs face a complex journey from diagnosis through post-treatment support. Patients typically progress through multiple providers and referrals before receiving treatment. As each of these episodes of care likely has its own associated cost or cost-sharing requirements, this can be financially burdensome for some patients. Additionally, treatment sites can be limited and dispersed geographically, which may require the patient to assume additional travel and lodging costs to access these types of treatments.
Prior to receiving treatment, patients often navigate a complex treatment approval process requiring input from multiple stakeholders, including payers, providers, and manufacturers. Patients navigating this process may also face additional non-medical cost burdens including travel and lodging expenses and loss of work productivity. These patients require a greater degree of assistance, care coordination, and navigation support to help them understand both these hurdles and the avenues of support from manufacturers and other entities.
Patient Coverage for CGT May Differ Greatly from National Coverage Breakdown
When designing patient support programs for CGTs, stakeholders should understand how the expected patient population receives coverage (e.g., commercial, Medicaid, Medicare) as well as the opportunities and challenges each of these insurance markets present in terms of providing support to patients. Given the patient demographics and coverage characteristics—compounded by unique access challenges—for CGT therapies, patient support programs must develop access and affordability solutions that are tailored to the specific needs and characteristics of the specific patient population.
For example, a CGT indicated to treat pediatric patients would need a patient support program built around the needs (and restrictions) of a patient population with greater Medicaid/CHIP enrollment than typical support programs for high-cost, innovative therapies that treat older patient populations that will more likely be covered by Medicare and commercial plans. Similarly, support focused on caregiver services will be critical in certain populations.
Innovative Patient Support Is Needed to Address Pre- and Post-Treatment Non-Drug Costs
When considering the cost of care for services outside of actual drug costs—such as testing and diagnostics, transportation and lodging, caregiver support, post-operative after treatment, and associated indirect costs such as loss of work and income—patients will need novel solutions that can provide support beyond traditional drug-specific support solutions.
Recently enacted policy proposals and increased flexibilities from federal regulatory agencies may offer new pathways for manufacturers to consider new types of support for patients that might help limit patient affordability challenges. For example, the Office of the Inspector General recently approved the legality of 1 manufacturer’s travel assistance program that includes financial support to some rural and indigent patients. Programs like these can cover some of the financial and out-of-pocket costs incurred by patients.
Considerations Moving Forward
With the FDA expecting to approve 10–20 CGTs per year by 2025,1 challenges to patient access and affordability could grow to the point that the total cost burden begins to exclude certain patients. Manufacturers should consider whether patient support services and new program designs can offer tailored solutions to unique and diverse patient populations.
Biopharmaceutical companies with CGT products in pipeline should consider patient support services that address the complexities of the patient journey for patients prescribed CGTs. The future design of assistance programs should include innovative solutions that address areas of greatest need along each step of the treatment journey.
Avalere is helping clients drive innovation through improved design and implementation of patient support programs, understand the patient population and payer mix, and explore the role other stakeholders can fulfill in partnerships with manufacturers to provide continuity of care to patients.
To receive Avalere updates, connect with us.
Notes
- Food & Drug Administration Press Announcement, January 15, 2019.






