CMS Expands Medicare Device Access Via New TCET Pathway
Summary
The new Transitional Coverage for Emerging Technologies pathway will provide Medicare coverage for up to five FDA-designated Breakthrough Devices per year.On August 7, the Centers for Medicare and Medicaid Services (CMS) released a final procedural notice outlining a new coverage pathway for Breakthrough Devices: the Transitional Coverage for Emerging Technologies (TCET). This announcement aligns with the agency’s commitment to ensuring Medicare beneficiary access to new technology and advancements that improve health outcomes and quality. The TCET pathway seeks to create a timelier path to CMS coverage determination for select Food and Drug Administration- (FDA) designated Breakthrough Devices, while also safeguarding patient safety.
The TCET pathway is intended to:
- Facilitate early and safer access to new technologies
- Increase transparency in coverage determination by reducing uncertainty and promoting early evaluation of benefits and risks of technology
- Identify evidentiary gaps and encouraging development within those areas
TCET Nomination and Eligibility
Manufacturers of Breakthrough Devices may self-nominate for participation in the pathway. CMS will accept non-binding letters of intent to nominate between 18-24 months in advance of expected FDA marketing approval. Manufacturers of Breakthrough Devices may self-nominate for participation in the pathway; CMS asks that manufacturers submit TCET pathway nominations approximately 12 months prior to expected FDA decision making on marketing authorization. TCET-eligible devices include those that are:
- FDA-designated Breakthrough Devices
- Determined to be within a Medicare benefit category (this excludes digital therapeutics)
- Not already the subject of an existing Medicare national coverage determination (NCD)
- Not otherwise excluded from coverage through law or regulation
Figure 1. TCET-Eligible Devices Are a Subset of FDA-Recognized Medical Devices and Breakthrough Devices
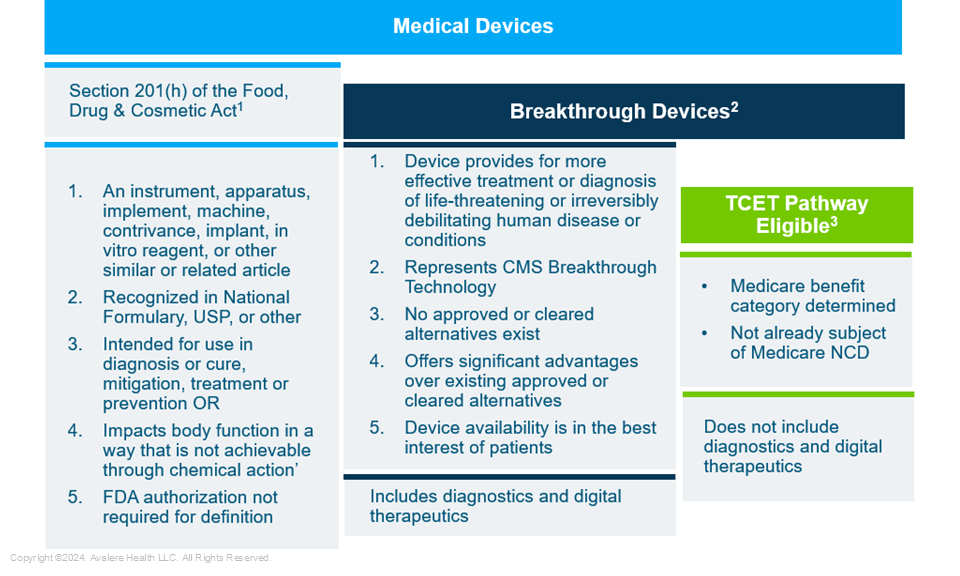
USP: United States Pharmacopeia
1.FDA Guidance on Medical Device Definition
2.Criteria for Breakthrough Devices defined by Final Guidance
3.Final Notice: Medicare Program; Transitional Coverage for Emerging Technologies
TCET Pathway to Coverage
CMS will accept up to five TCET candidates per year, with the goal of formalizing an NCD within six months of the technology receiving FDA market authorization. The cap on TCET acceptances per year limits the number of Breakthrough Devices that may receive prioritized coverage and may create a tiered system of early access to emerging technologies. The agency noted that evidence development will be based upon a more transparent framework; however, this remains to be seen as it expects to release detailed guidance on this framework in the future.
There are seven distinct phases of TCET:
- Letter of Intent: Informal and non-binding.
- Evidence Preview: Contractors will have an early look into available and missing evidence, as well as coverage pathways for a technology
- Marketing Authorization: Granted by the FDA
- Evidence Development Plan: Developed by manufacturers to address evidentiary gaps identified in the preview in collaboration with CMS and the Agency for Healthcare Research and Quality
- NCD Designation and Transitional Medicare Coverage: CMS issues product-specific NCD within 6 months of FDA marketing authorization; CED and transitional coverage period begins
- Continued Coverage: TCET extends national coverage for devices for an indefinite time period until sufficient evidence has been collected to inform CMS coverage decision making, which CMS expects to last more than five years
- Post-TCET Coverage and Review: Following manufacturer evidence development and analysis, CMS will re-review evidence and issue one of the following: NCD without evidence development, NCD with CED, non-coverage NCD, MAC discretion.
Industry Urges Broader Reach
In comments on the proposed rule, advocates and manufacturers expressed concern about the limit of five TCET awardees per year, indicating that this may not make a significant difference given the large bottleneck of Breakthrough Devices. Upon publication of the final procedural notice lawmakers, industry representatives, and manufacturers have recognized CMS’s efforts to prioritize access to Breakthrough Technologies, but expressed hope that the number of devices granted TCET status per year and the scope of devices/technologies eligible (e.g., diagnostics, digital therapeutics) might expand in the future.
TCET Pathway Raises Strategic Questions for Manufacturers
While the TCET pathway presents the opportunity for swifter Medicare coverage and broader opportunities for CMS engagement and evidentiary review, uncertainties remain. Manufacturers will require a strategy for the TCET nomination and application process, engagement with CMS, data collection, and reporting of data collected for final coverage decision-making.
With demonstrated expertise in medical device access and Medicare coverage, Avalere can partner with manufacturers in understanding potential eligibility for TCET, assessing the decision to apply, and generating evidentiary data collection and facilitating engagement with CMS. Connect with us today.







