Ensuring Patient Access by Navigating Decisions at FDA and CMS
Summary
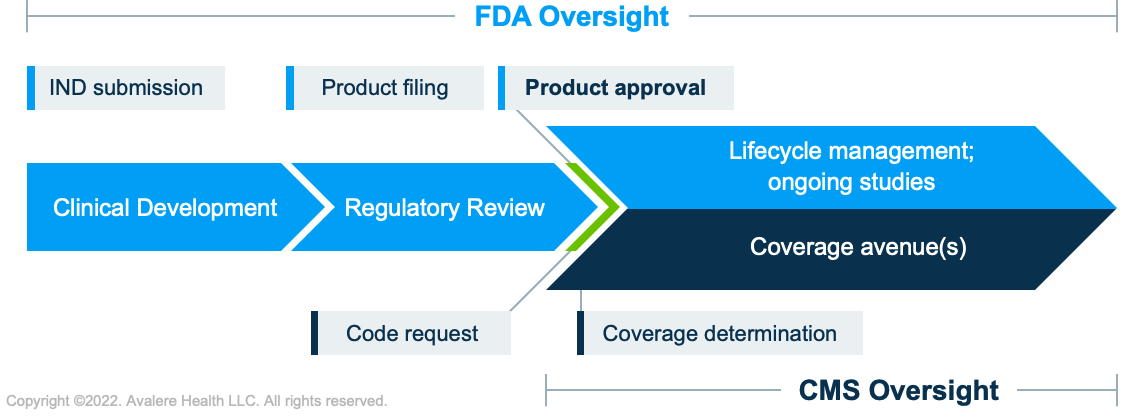
The FDA and CMS have historically had complementary roles. Recent activity highlights how their unique functions affect access to medicines.Together, the Food and Drug Administration (FDA) and Centers for Medicare and Medicaid Services (CMS) maintain US regulatory authority over medical products looking to gain marketing clearance and be covered in public insurance programs. The agencies both contribute to the same overall charge: to improve the health of patients through the availability of and access to safe and effective medical products. However, the 2 agencies share complementary but distinct goals, and their approaches to oversight during medical product life cycle are unique (see Figure 1).
What is Each Agency’s Responsibility?
Roles, responsibilities, and decisions of each agency are rooted in their respective legislative mandates. In the case of FDA, the agency’s mandate is to determine if a product is “safe and effective” to enter the marketplace. In the case of CMS, it is to determine if each FDA-approved product is “reasonable and necessary” for the care of Medicare patients.
While the mandates and roles are wholly different for each agency, the layered decision making throughout the life cycle of a product plays a key role in ensuring the right products reach the right patients and there is more benefit than harm.
Other factors beyond agency discretion can also play into patient access. These include FDA advisory committee recommendations, ongoing post-marketing requirements and studies, commercial payer policies on product coverage, and, ultimately, healthcare provider adoption of the product in question.
| FDA | CMS | |
|---|---|---|
| Objective | Permit the interstate commerce of safe and effective medical products. | Provide access to effective medicines for certain eligible American populations. |
| Initial Value Assessment | To be marketed, product must demonstrate safety and efficacy based on requirements of approval type. Traditional approval requires substantial evidence to support that the effect on a clinical endpoint predicts clinical benefit. Accelerated approval requires evidence that demonstrates an effect on a surrogate endpoint is reasonably likely to predict clinical benefit. | To be covered, product must be approved by the FDA and meet the CMS’s definition of reasonable and necessary. |
| Ongoing Value Assessments | Outcomes monitored or confirmed using post-marketing studies or confirmatory studies. | The CMS may issue national coverage determinations that require evidence development as a condition of coverage (i.e., Coverage with Evidence Development). |
FDA Approval Pathways Have Significant Implications in Recent CMS Decisions
As biomedical innovation has progressed to allow for the targeting of increasingly rare and complex disease states, the FDA has adapted risk/benefit assessments for marketing approval of treatments for these serious or life-threatening diseases. Subsequently, the decisions by the 2 agencies have increasingly intersected. For instance, the recent CMS National Coverage Determination on monoclonal antibodies for Alzheimer’s disease requires additional evidence to confirm a product’s clinical benefit that is above what is necessary for the FDA’s Accelerated Approval. The CMS will require coverage with evidence development for all products approved that fall within this therapeutic class, but those approved using the traditional approval method are not subject to the same level of scrutiny for confirming clinical benefit. This action may signal a change in how the CMS approaches FDA-approved products and indicates that each agency may view the same data differently for approval or coverage decisions, respectively.
The complementary but distinct roles of the FDA and CMS may continue to garner public attention, especially at a time when novel biotechnology innovations enable treatment options for more serious or rare diseases. Additionally, Congress may enact modifications to the regulatory process and timing for sponsor evidence generation under accelerated approval, as well as for other special FDA designations, as part of the user fee reauthorization process. Part II of this Insight series provides a more nuanced account of how FDA and CMS oversight has been shaped over the years, as well as the legislative vehicles that may codify future FDA-CMS collaborative efforts.
Avalere is well positioned with policy, regulatory, and market access expertise to help stakeholders strategize and prepare for ongoing FDA and CMS engagement and landscape shifts that may impact evidence generation plans, reimbursement potential, and ultimately, patient access to medicines.
To learn more about how Avalere can support you with these efforts, connect with us.




