Generic Drug Placement on Part D Generic Tiers Declines Again in 2021
Summary
A new analysis from Avalere finds that in 2021, Medicare Part D plans place generic prescription drugs on generic tiers 45% of the time, a decrease from 64% in 2016.Medicare Part D plans have the flexibility to design drug formularies and structure tiers as long as they meet the formulary design requirements of the Centers for Medicare & Medicaid Services (CMS). Since 2017, plans have had the option to include a greater proportion of generic drugs on the non-preferred tier if they replace the “non-preferred brand” tier with a “non-preferred drug” tier. Prior Avalere analyses assessed the outcome of this policy change, finding that over time Part D plans have increasingly added more generic drugs from generic tiers onto the “non-preferred drug” tier.
This analysis from Avalere, which has been run annually and with the same methodology since 2016, again finds that Part D plan sponsors are increasingly placing generic drugs on higher tiers over time (Figure 1).
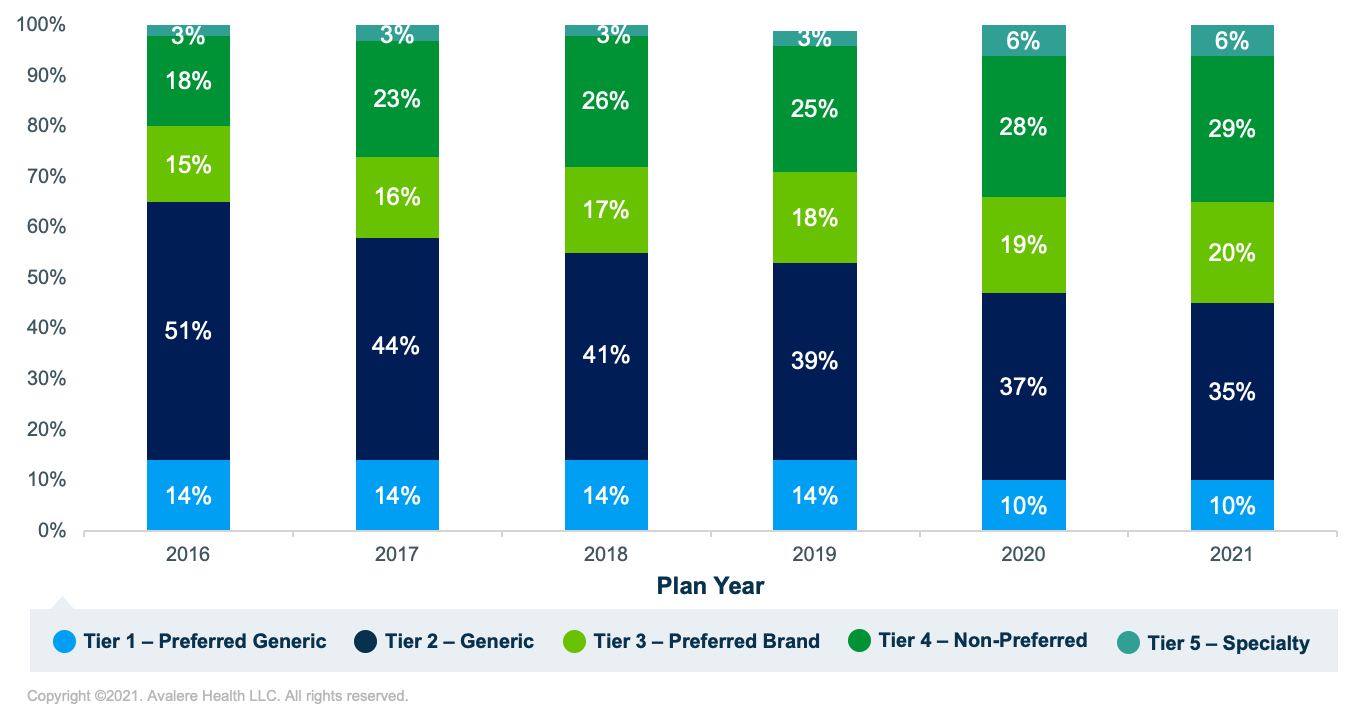
At the same time, the proportion of generic products placed on non-generic tiers (i.e., any tier not designated specifically for generics) has increased over time (Figure 2). 2020 was the first year since the launch of the Part D program that generics were placed on non-generic tiers more often than on generic tiers, and this figure grew in 2021, with 55% of covered generic products now placed on non-generic tiers. This is a 19-percentage-point increase from 2016, when generic products were placed on non-generic tiers only 36% of the time.
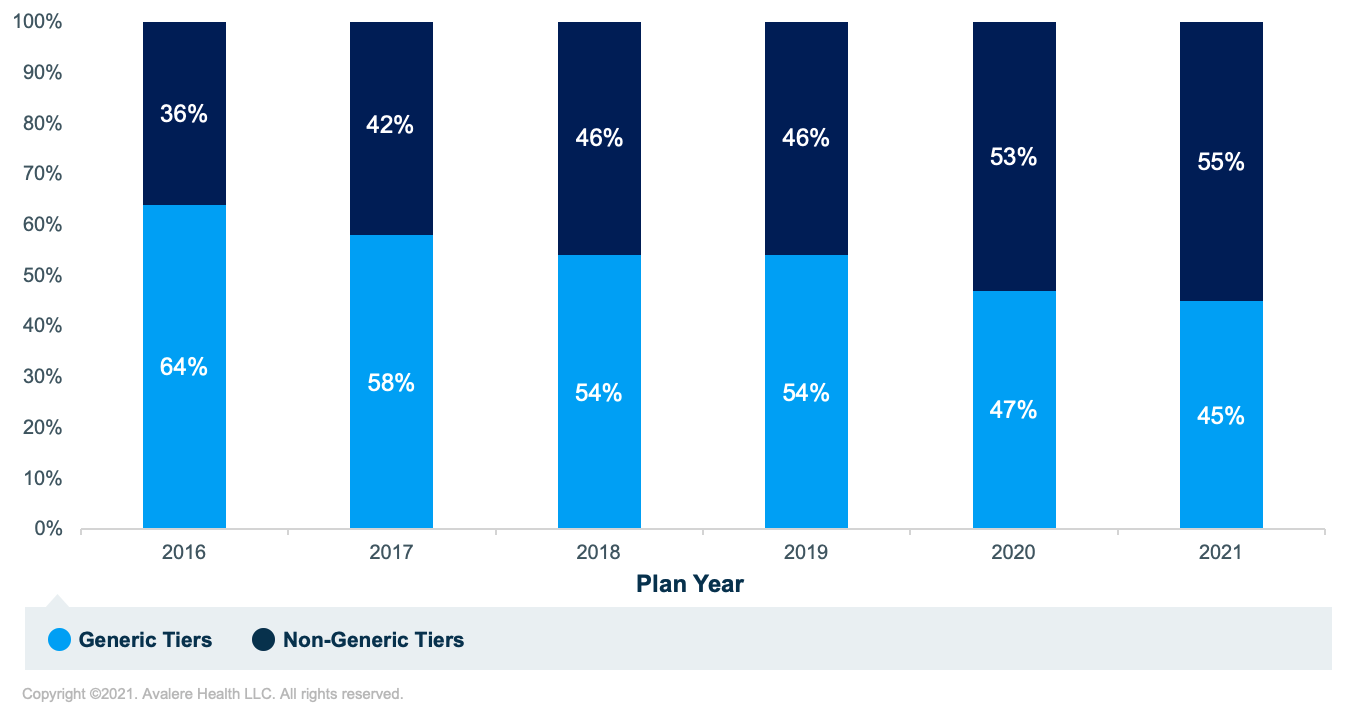
Under Medicare Part D benefit design requirements, beneficiaries generally pay more cost sharing for drugs on higher tiers (i.e., Tiers 3 and 4) than those on lower tiers (i.e., Tiers 1 and 2). Therefore, when generic products are placed on non-generic tiers, patients often pay more out of pocket for these medications than if they were placed on generic tiers.
Funding for this research was provided by the Association for Accessible Medicines. Avalere Health retained full editorial control.
Methodology
Avalere analyzed CMS public use files (PUFs) with Medicare Part D formulary and benefit design information for 2016–2021. The study used the brand/generic indicator assigned to drugs in the formulary data. Part D plans name and define their tiers in a variety of ways. For consistency, this analysis aggregated different tier names into 6 tier categories: generic, preferred generic, preferred brand, non-preferred brand, non-preferred drug, and specialty. For all years, Avalere used actual plan formulary design and tiering information for generic drugs from the CMS’s PUFs.







