Implications of Self-Administered Drug List Determinations on Access
Summary
SAD list placement will inform the channel that providers and patients use to access a therapy.Background
Medicare Administrative Contractor (MAC) self-administered drug (SAD) exclusion lists may affect Medicare benefit categorization for drugs and biologics, which has implications for manufacturers, providers, and patient access. Medicare typically covers drugs under one of two benefit categories, Part B or Part D, which generally depends on the route and site of administration, with some exceptions defined in statute. Under Medicare fee-for-service (FFS), MACs have the authority to evaluate drug and biologic product criteria for coverage and payment. One such tool is the SAD exclusion list. The SAD exclusion list clarifies benefit assignment for certain products, often subcutaneous therapies, that may be administered incident to a physician visit and/or self-administered. Treatments placed on SAD exclusion lists by MACs are deemed not eligible for coverage within that MAC’s jurisdiction but may be covered under the beneficiary’s Part D benefit. There are multiple regulatory and coding decisions that manufacturers may make that inform if and how a product may be placed on the SAD list.
SAD Exclusion List Criteria
SAD exclusion lists are specific to each MAC and may vary from one jurisdiction to another, depending on how each MAC has structured its process for reviewing drugs. However, MACs use standard criteria set forth by CMS when evaluating drugs for placement on SAD exclusion lists, thus classifying them as not appropriate for Part B coverage. Manufacturers should account for these criteria as 4 key factors to consider in product development and understand the implications of product characteristics on MAC coverage decisions.
These criteria include:
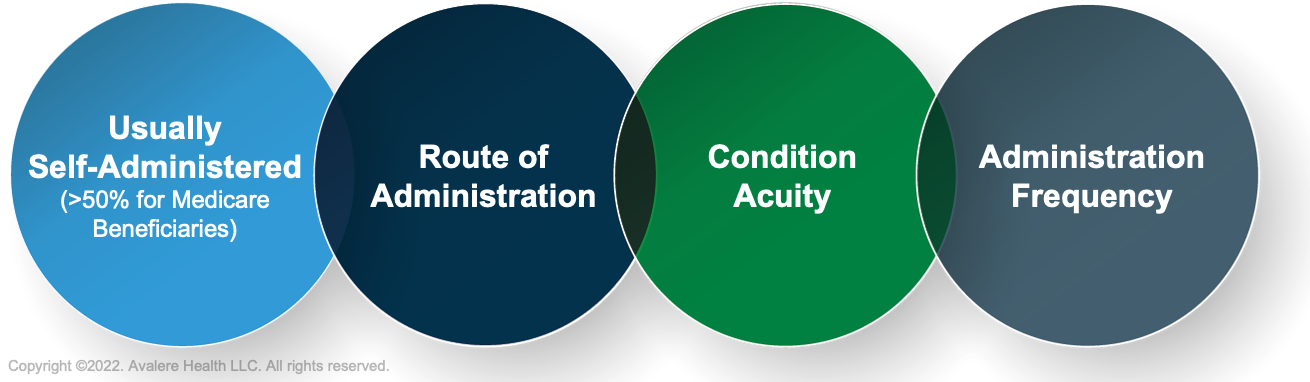
Source: Centers for Medicare and Medicaid Services. Medicare Benefit Policy Manual
As part of the Calendar Year 2024 Medicare Physician Fee Schedule (MPFS) Notice of Proposed Rulemaking, CMS solicited comments through a request for information (RFI) on the existing Self-Administered Drug (SAD) exclusion list policy and the process for determining if a drug is usually self-administered by the patient. Although the 2024 final and 2025 proposed MPFS rules did not include changes to the SAD list determination process, monitoring future developments in this area could influence how stakeholders choose to engage MACs throughout the determination process.
Proactively Addressing Implications
Manufacturers may seek to engage with and provide additional information to MACs to aid in their interpretation of the criteria and data during the evaluation process. SAD exclusion list determinations will likely impact provider billing and reimbursement downstream, so manufacturers may need to develop education and engagement efforts to inform of potential changes in access to and reimbursement upon initiation of the notification period.
Additionally, the determination to list a drug as self-administered can significantly impact patient access and affordability. Patients who previously received a SAD-listed drug through their Part B FFS benefit would, following the SAD-list determination, need to obtain the medication through a pharmacy. This change may result in considerable variation in patient cost-sharing, depending on their Part D plan benefits and the specific cost of the product formulations.
Understanding the requirements for and potential challenges of a SAD exclusion list determination is critical for manufacturers as it may drive the commercial and patient access environment for their therapies. Avalere has deep expertise and experience assessing MAC coverage processes and developing strategies to engage MACs and stakeholders to support access across the product lifecycle.
Contact Avalere to learn more about how our experts in Medicare Part B and Part D coverage and reimbursement and MAC engagement can leverage Avalere’s proprietary Medicare claims data to inform a strategy positioning you to effectively engage with MACs.







