Manufacturer’s Compassionate Use Policies: Companies with Posted Policies More Than Doubled Since September 2016
Summary
The 21st Century Cures Act requirement to post compassionate use policies may explain increase.Called “compassionate use”, “preapproval access,” or “expanded access”, stakeholders have increasingly debated the issue of whether and how best to provide patients access to investigational products outside of clinical trials (i.e., drugs and biologics not-yet-FDA approved for any use; see Avalere 360® Focus Report here).
A physician seeking an investigational product for a patient first contacts the manufacturer of that drug to request access. Manufacturers are under no obligation to provide their investigational products and while some agree to give access, others may deny a request for a number of reasons (e.g., lack of sufficient supply of drug, the patient can join a clinical trial, the drug is likely not appropriate for the patient, or the manufacturer can give no reason at all).
Patients and their physicians have expressed frustration at locating the appropriate contact and information on how to request compassionate use access; understanding manufacturer’s policies for granting access; finding the status of their request; and determining if a company grants access at all. Having a transparent compassionate use policy that patients and their physicians can easily find would be expected to help to alleviate these issues. Last Fall, we assessed the then-current state of transparency of manufacturer’s compassionate use policies and in this update, following the passage of the 21st Century Cures Act in December 2016, we revisit this assessment to investigate if and how the landscape has changed.
In September 2016, only 19% of Companies Posted Compassionate Use Policies
In September 2016, we reviewed the websites of 100 publicly traded pharmaceutical and biotechnology companies, 25 “large” (> $10B market cap), 27 “medium” ($1.5B–$10B market cap), and 46 “small” (<$1.5B market cap).
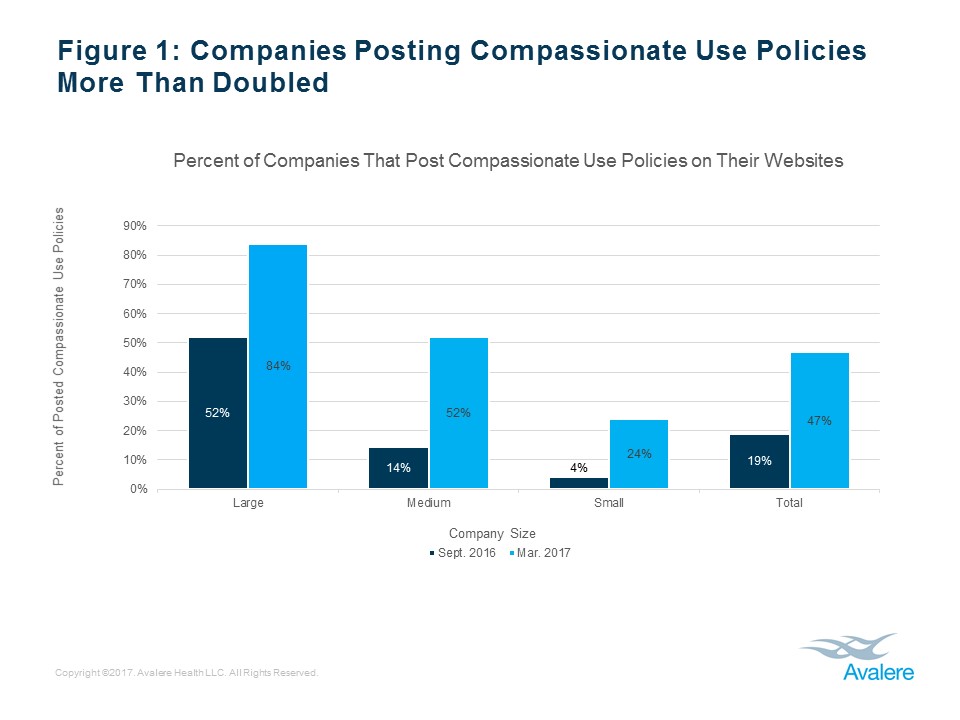
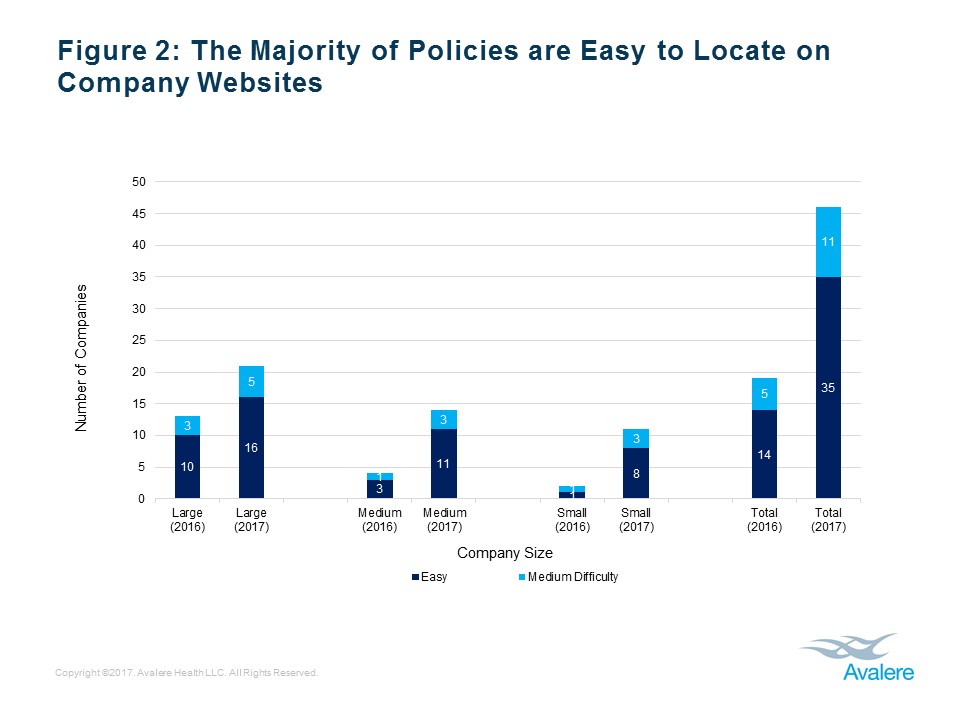
We found that of the 100 companies reviewed, 19 companies (19%) included a compassionate use policy on their website. Large companies were more likely to post a compassionate use policy than smaller companies, and almost 75% of the posted compassionate use policies were easily accessible on companies’ websites, although the website placement varied. See Figures 1-3, below (for the full report, including methodology, see Avalere press release, “Few Manufacturers Publicly Share Policies for Granting Patient Access to Investigational Products“).
The 21st Century Cures Act Passes with Compassionate Use Policy Transparency Requirements
In December 2016, Congress passed the 21st Century Cures Act which includes a provision requiring manufacturers to publish the following: (1) contact information for the manufacturer or distributor to facilitate communication about requests for access to unapproved medicines; (2) procedures for making compassionate use requests; (3) the general criteria considered for evaluating requests; (4) the length of time anticipated to acknowledge receipt of requests, and (5) a hyperlink or other reference to any clinical trial record about expanded access for the drug. Manufacturers were required to comply with this Expanded Access Policy provision either by February 11, 2017 (i.e., 60 days after enactment), or when the manufacturer first initiated a phase 2 or phase 3 study of the investigational drug.
More Than Twice the Companies Now Post Compassionate Use Policies
We revisited the same companies (now 98 given mergers) in March 2017, to determine how many additional companies posted compassionate use policies and to investigate if the posted policies from September 2016 had changed. We found that the number of companies posting a compassionate use policy more than doubled, from 19% to 47%, and 84% of large companies now post policies. See Figure 1, below.
Consistent with our previous findings, about 75% of the total compassionate use policies were easily located on the company’s website. See Figure 2, below.
We continue to find that website placement varies, with 66% of compassionate use policies located in website sections related to research and/or development, products, pipeline, or clinical trials. See Figure 3, below.

Compassionate Use Policy Transparency Outlook
Passage of the 21st Century Cures Act likely explains the increase in posting of manufacturer’s compassionate use policies. For some companies on our list, it may be that they are not required to (yet) comply with the Expanded Access Policy provision found in the Act (e.g., do not yet have an investigational product in phase 2). However, we recommend all companies developing investigational products create and post a compassionate use policy.
We anticipate that pressure on manufacturers to maximize patient access to investigational products, help patients and their physicians understand how to request access, and provide timely responses to requests will not subside. While the increase in transparent compassionate use policies we see is very encouraging, there is still work to be done with regard to consistent use of terminology and website placement (and ease of finding the policy), which will further reduce burdens to patients and physicians looking to understand how to request access for a specific investigational product.
While it is currently unclear whether FDA has plans to enforce the Expanded Access Policy requirement found in the 21st Century Cures Act, at a minimum, manufacturers should have a simple policy on their website (even if a policy of “no access at this time”) and always include a point of contact to provide greater transparency and access for patients and their physicians.
January 23, 11 AM ET
Learn More


