Rare Disease Advisory Councils: Opportunities for Engagement
Summary
Stakeholders should seek to engage with RDACs to develop a better understanding of rare disease care access, treatment, and populations in different states.Background
About 30 million people in the United States suffer from rare diseases and 95% of rare diseases lack a Food and Drug Administration-approved treatment. There are over 10,000 types of rare or genetic diseases, which makes it difficult for government officials and policymakers to understand the unique needs of the rare disease community.
To help rare disease communities navigate these challenges, many state legislatures have created Rare Disease Advisory Councils (RDACs), which help states refer rare disease patients to specialists, evaluate treatments, improve awareness of rare diseases, and create strategies that stakeholders (e.g., health providers, payers, advocacy organizations) can implement to improve the quality of care and health outcomes for patients with rare diseases. There are currently 28 RDACs, with the first created in 2015 in North Carolina, in addition to one rare disease advisory working group in New York. In September 2024, California became the most recent state to sign an RDAC into law
Figure 1: Map of RDACs by Year Established
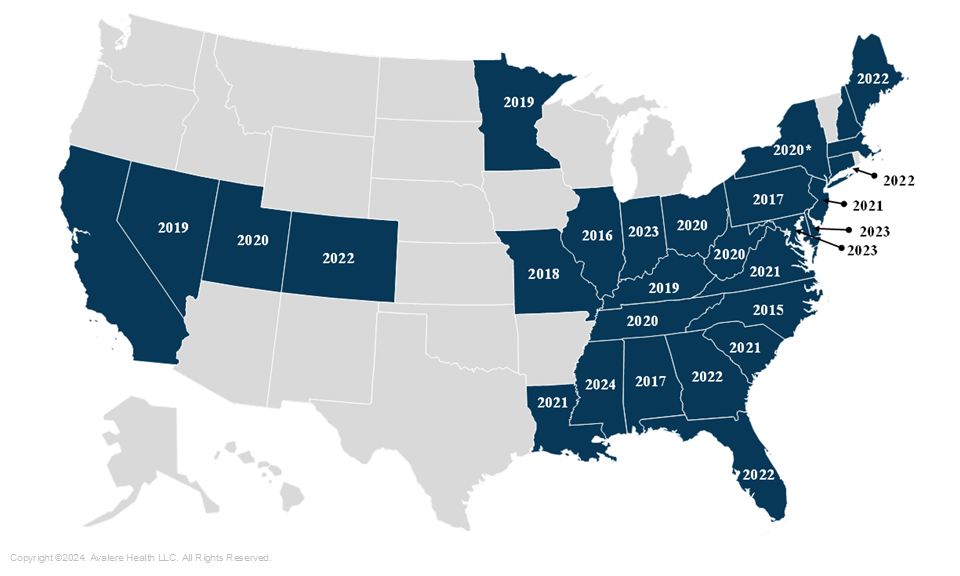
*New York Rare Disease Working Group is not an official RDAC.
There are substantial differences across RDACs, with each state determining the composition, function, responsibilities, state funding mechanism, and size of their council. RDAC members include a variety of rare disease stakeholders, including patients, patient advocates, providers, caregivers, researchers, biotech industry representatives, state government officials, and health insurance representatives.
RDACs often work in conjunction with rare disease organizations such as patient advocacy groups, the National Organization for Rare Disorders, and other stakeholders in the rare disease community (e.g., academic medical centers). These partnerships help RDACs identify state-specific issues (e.g., barriers to treatment) for the rare disease community and enable them to provide comprehensive recommendations through engagement with relevant stakeholders. Given RDACs’ limited resources, partnering with different stakeholders can also provide funding for various programs to assist patients with rare diseases and execute councils’ goals.
Function of RDACs
Given the number of rare diseases, it is difficult for government officials and policymakers to understand the unique needs of the rare disease community. This can hinder treatment options, result in high out-of-pocket costs, limit access to specialists, and cause delays in diagnosis and treatment. In evaluating and addressing state-specific barriers to care, RDACs can bridge the gap between legislators, health departments, and the rare disease community.
RDACs conduct surveys to assess the needs of the rare disease community, publish resources for patients and families, and consult with experts to improve access and quality of care for patients, among other activities. Some RDACs also hold public meetings on their current priorities.
RDAC Limitations
Although RDACs can be valuable resources for understanding state-specific rare disease landscapes in the state, they do have limitations. RDACs are advisory bodies, meaning that they can provide recommendations to policymakers, but do not have authority to set policy. Furthermore, the amount of funding and resources RDACs receive differs across states, as many RDACs do not receive state appropriations. It is important for stakeholders to understand the differences in funding, activity, and organization between RDACs in different states to plan engagement opportunities. Additionally, the structure and goals of RDACs can be different across states, which make it imperative for stakeholders to understand the resources, capabilities, and support that an RDAC has prior to engagement.
Opportunities for Engagement
Engaging with RDACs can help stakeholders better understand the patient demographics and barriers to care for rare disease in a particular state. Furthermore, RDACs often have strong relationships with prominent rare disease medical centers and state-based patient organizations, which could help stakeholders identify partners to engage and improve health outcomes for patients with rare diseases. Additionally, through communications with RDACs, stakeholders may have opportunities to help promote the creation of more RDACs and assist in policymaker education to understand the intricacies of the rare disease diagnostic odyssey and patient journey.
Avalere supports manufacturers, patient advocacy groups, payers, and providers in reaching out to RDACs to gain a better understanding of the needs of the rare disease population in different states. To find out more about RDACs, how to engage with different states’ councils, and learn how Avalere can support you, connect with us.
January 23, 11 AM ET
Learn More



