Recent Federal Rule Could Undermine Some Patient Support Programs
Summary
The Center for Medicare & Medicaid Services (CMS) recently issued its proposed Notice of Benefit and Payment Parameters (NBPP) for the 2021 plan year. The proposed rule would significantly expand commercial payer flexibility to not count manufacturer copay support toward deductibles or out-of-pocket (OOP) maximums.Proposed Changes
On January 31, CMS released the proposed NBPP for the 2021 plan year. The proposed NBPP would revise the regulatory definition of cost sharing that applies to all commercial plans (insured and self-funded) and issue clarifications to provide additional flexibilities for accumulator programs.
- Definition: Proposes to redefine the regulatory definition of cost sharing to allow payers to exclude any form of direct manufacturer cost sharing support for any drug (regardless of generic alternatives) from counting toward member spending limits.
- Application: Clarifies that all commercial plans (insured and self-insured) would be able to use accumulator programs to exclude manufacturer copay support if those practices don’t violate state law.
- Notice: Proposes to require plan sponsors to be fully transparent with current and prospective members around the existence of accumulator programs and how those programs could impact OOP liability. Disclosures would need to be prominently displayed in websites, brochures, plan summary documents, and any other plan collateral.
Background
In recent years, CMS has pursued policy changes that would allow issuers and plan sponsors to directly counter drug manufacturer copay support. The CMS has noted that this flexibility is needed to allow payers to incent the use of generic alternatives and to help plan sponsors reduce spending, especially on branded prescription drugs. In last year’s notice, the CMS sought to do this by amending cost-sharing application guidance to authorize plan sponsors not to count manufacturer copay support whenever a medically appropriate generic was available.
However, the CMS’s recent actions sparked confusion as to whether it may have actually reduced accumulator flexibility for self-insured group health plans—limiting accumulator programs to only brands with generic alternatives. In addition, there was concern that requiring plans to count copay coupons toward spending limits where there wasn’t a generic could create compliance issues for HSA-eligible high deductible and consumer-directed health plans.
In late 2019, therefore, the CMS delayed enforcement of federal patient protections around accumulator programs and announced it would provide clarification in the 2021 NBPP. The following issues should be top of mind for stakeholders with respect to the CMS’s new accumulator proposals.
Implications
These proposed changes raise several key considerations for stakeholders:
- If finalized, this policy could cause additional financial access challenges for patients, especially mid-year when patients may be mid-treatment or may need follow-up care to ensure treatment effectiveness and disease management.
- Accumulator programs can result in mid-year affordability challenges since they impact how quickly patients may reach their deductible and OOP limits. Since many manufacturer copay assistance programs have total annual assistance eligibility limits, patients more often reach these support caps before reaching their plan’s spending limits.
- A sudden increase in cost-sharing burden could lead to treatment abandonment or delay which could affect treatment effectiveness, outcomes, and overall condition management.
- To illustrate, consider an example patient OOP cost scenario:
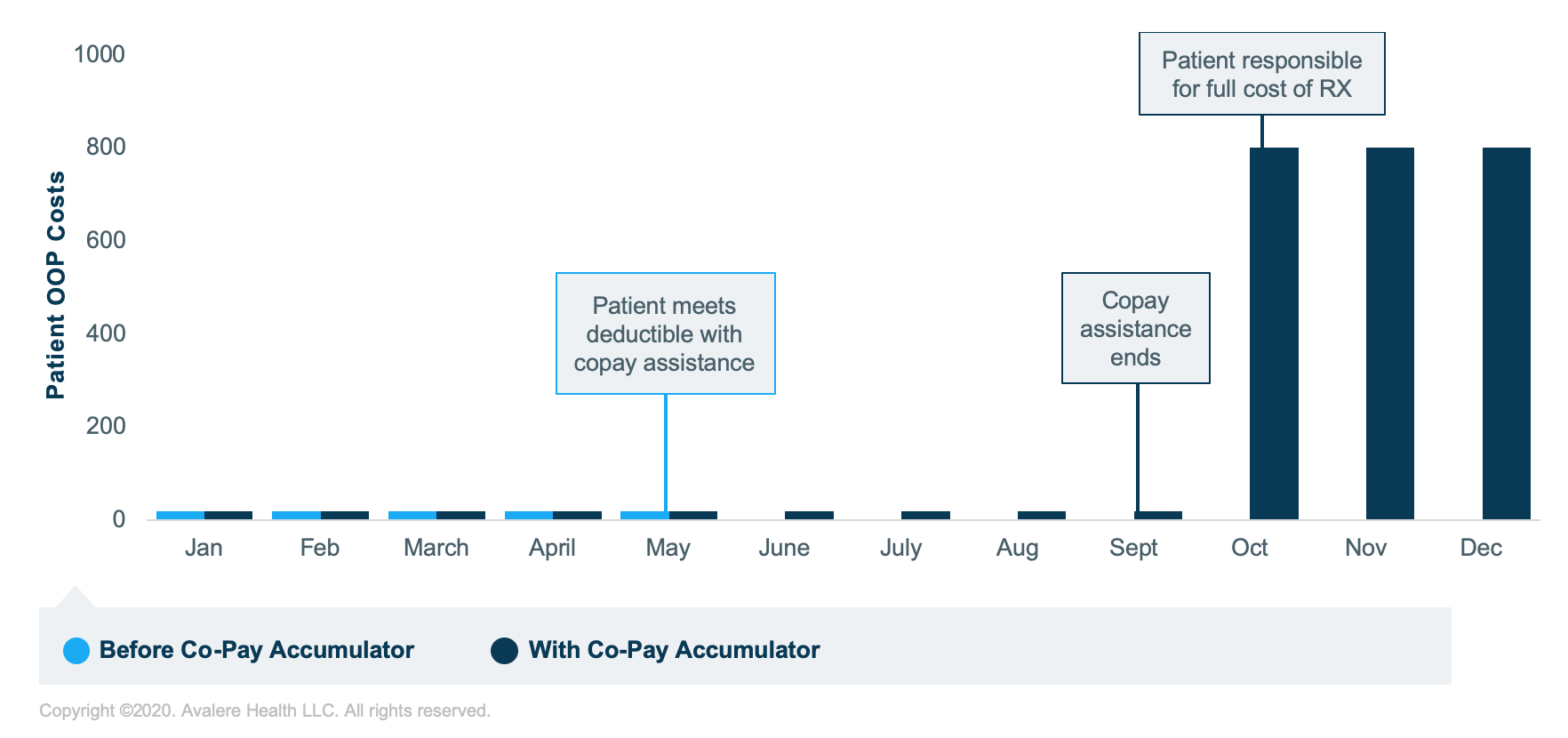
- An expansion of copay accumulator flexibilities may lead to these programs being more widely used in self-insured market and expanding into the insured individual and group markets, and for new therapeutic areas or drugs. Manufacturers may need to reconsider how their cost-sharing support programs are structured to mitigate gross to net impacts.
- The impacts are unlikely to be felt uniformly across patient populations. Patients managing complex or rare conditions like cancer, rheumatoid arthritis, multiple sclerosis, HIV, or hepatitis C may be most impacted.
Avalere is engaging on the impacts of the proposed changes and how the changes could affect patient access and support strategies. To learn more about our work on accumulators and patient access programs, connect with us.
Find out the top 2020 healthcare trends to watch.
* Assumptions: Drug cost per month pre-deductible: $800; deductible/MOOP: $4,000; coupon card available ($5 patient OOP and $7,000 cap).







