Therapeutic Vaccines Raise Regulatory, Policy Pathway Questions
Summary
In light of the growing therapeutic vaccine pipeline, policymakers are starting to define coverage, access, and reimbursement pathways.Vaccines are conventionally administered prophylactically to prevent disease and associated morbidities. In recent years, manufacturers have increasingly used vaccine platform technology to develop therapeutic applications that treat or prevent disease exacerbation rather than prevent onset. In response to pipeline therapeutic vaccine progress toward late-stage development, policymakers are beginning to define policy and access pathways for these novel products. Recent policy action suggests that these products may move through drug-like access pathways, in contrast to currently available preventive vaccines and passive immunizations like preventive monoclonal antibodies.
Background
Therapeutic vaccines typically stimulate a recipient’s immune system to slow or stop chronic disease progression or target cancer cells. Unlike preventive vaccines, therapeutic vaccines may be customized to respond to specific mutations. There are myriad therapeutic vaccines currently in development that target a range of infectious diseases, chronic conditions, and cancers (see Table 1).
| Indication | Development Phase |
|---|---|
| Acute Myelogenous Leukemia | 3 |
| Mesothelioma | 2 |
| Melanoma, Colorectal Cancer, Non-Small Cell Lung Cancer | 2 |
| Chronic Hepatitis B | 2 |
| Multiple Myeloma | 1/2 |
| Ovarian Cancer | 1/2 |
| Type I Diabetes | Preclinical |
Coverage of and access to preventive vaccines and therapeutic drugs are determined by decisions made along distinct pathways (Figure 1). These pathways directly or indirectly influence product pricing, coding, coverage, and reimbursement. Federal law mandates coverage without cost-sharing for Advisory Committee on Immunization Practices (ACIP)-recommended vaccines across markets; there is no similar statutory requirement for drugs, though the Affordable Care Act mandates that plans cover some drugs to comply with essential health benefits.
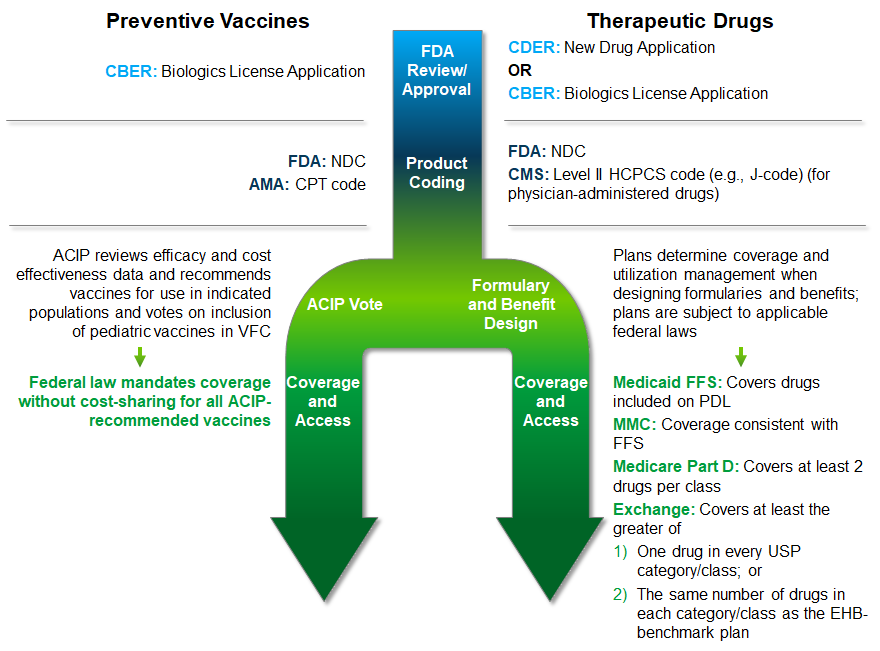
*Passive immunizations, including certain preventive monoclonal antibody products, may deviate from these pathways.
ACIP: Advisory Committee on Immunization Practices; AMA: American Medical Association; CBER: Center for Biologics Evaluation and Research; CDER: Center for Drug Evaluation and Research; CMS: Centers for Medicare & Medicaid Services; CPT: Current Procedural Terminology; EHB: Essential Health Benefit; FDA: Food and Drug Administration; FFS: Fee-for-Service; HCPCS: Healthcare Common Procedure Coding System; MMC: Medicaid Managed Care; NDC: National Drug Code; PDL: Preferred Drug List; USP: United States Pharmacopoeia; VFC: Vaccines for Children
Therapeutic vaccines leverage preventive vaccine platforms, and it is unclear which approval processes and policy frameworks will determine payer coverage and patient access. As these products approach late-stage development and market availability, policymakers are starting to provide clarity, creating opportunities for stakeholders to shape the therapeutic vaccine marketplace.
Policy Developments
Recent policy developments indicate therapeutic vaccines may be considered more like drugs than their preventive counterparts.
- Medicaid Drug Rebate Program (MDRP): In a May 2023 proposed rule, CMS proposed a definition of “vaccine” that would allow therapeutic vaccines to be included in the MDRP and subject their manufacturers to mandatory rebates. The rule defines “vaccine” to mean a “prophylactic” product that induces “active, antigen-specific immunity,” thus differentiating between conventional preventive vaccines and therapeutic vaccines.
- ACIP: Policymakers are increasingly considering passive immunizations, including certain prophylactic monoclonal antibody (mAb) products, to be similar to vaccines in some scenarios. On August 3, ACIP voted to recommend nirsevimab, a pediatric mAb for respiratory syncytial virus prevention, and to add nirsevimab to the VFC program. These decisions ensure coverage for nirsevimab without cost-sharing.
- American Medical Association Current Procedural Terminology (CPT) Editorial Panel: In May, the panel assigned nirsevimab a CPT code for “Immunoglobulin, Serum, and Recombinant products” rather than for “Vaccines, Toxoids,” differentiating it from preventive vaccines and potentially affecting how it is covered and reimbursed for all providers.
If therapeutic vaccines are subject to separate drug policies or coded separately from preventive vaccines, their coverage without cost-sharing may not be guaranteed across all markets.
Key Considerations for Manufacturers
As therapeutic vaccines for cancers and other indications approach market availability, manufacturers should consider how various regulatory and policy mechanisms could affect provider reimbursement, patient access, and overall utilization and market share. Key questions to consider include:
- What are the FDA’s expectations for evidence generation, given altered safety, efficacy, and risk/benefit profile considerations for therapeutic vaccines?
- How will therapeutic vaccines be covered by public and private payers? How could this affect provider reimbursement and patient cost-sharing?
- How could provider reimbursement for therapeutic vaccines affect where patients can receive them?
- Which provider types (e.g., physician, physician assistant, advanced practice registered nurse) will be authorized to administer therapeutic vaccines?
Answers to these questions could influence evidence requirements and policy pathways for therapeutic vaccines as well as preventive vaccines and vaccine-like products. As federal policymakers develop regulations and guidelines to differentiate products developed on vaccine platforms, manufacturers should consider how evolving policy infrastructure will shape their policy, evidence, and access strategies.
How We Can Help
Avalere’s vaccine policy and market access experts can help stakeholders navigate the regulatory and market dynamics unique to therapeutic vaccines. To learn how Avalere can support you, connect with us.





