Using Diagnostics to Mitigate the COVID-19 Public Health Emergency
Summary
Mitigating the public health emergency (PHE) caused by SARS-CoV-2 requires a multifaceted approach.While vaccines and therapeutics are progressing through the development and approval process, distribution and uptake challenges continue to influence the speed at which either contribute to a potential nationwide solution. Concurrently, development of other tools in the toolbox—such as diagnostics—must continue. These need to be supported and incorporated into the healthcare system to reduce the spread of SARS-CoV-2, diagnose people appropriately, enable treatment of those infected with the virus, and support the recovery for those who have been infected. As the continuum of COVID-19 treatment strategies evolves, along with an expanding understanding of the epidemic, so too will the potential impact and role of the various types of diagnostics. Strategies to optimize patient access to different tests will also need to account for this evolution to ensure products are able to be accessed at the right point in the continuum and by the right patients.
There are 3 main types of in vitro diagnostics used to mitigate the COVID-19 PHE, including:
- Diagnostic tests that detect the presence of an active SARS-CoV-2 virus infection, either through testing for viral nucleic acids (through polymerase chain reaction–based tests) or viral proteins (through protein-based antigen tests)
- Serology tests that determine whether someone has been previously exposed to the virus by testing levels of antibodies present
- COVID-19 management tests that are used to detect the impacts of the virus (e.g., inflammatory markers) on those who have contracted it
* Denotes products that also aid in prevention.
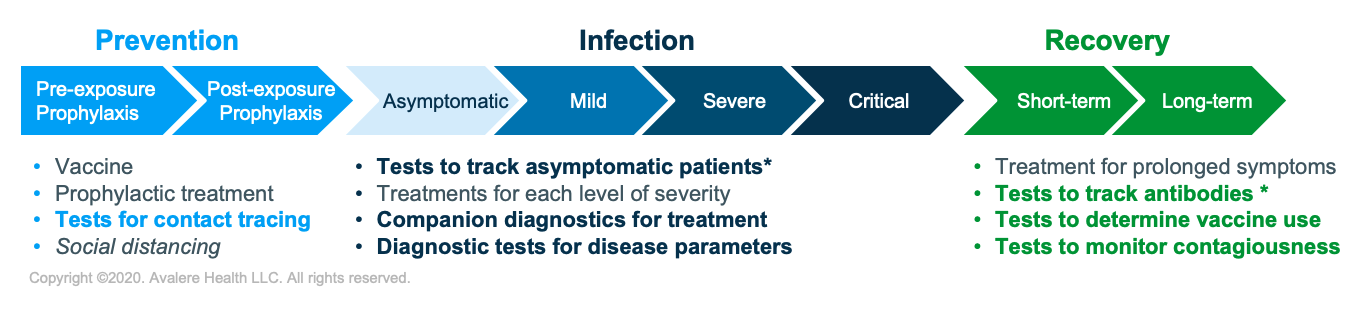
Each type of COVID-19-related in vitro diagnostic will continue to take on a variety of roles as part of the COVID-19 toolbox throughout the various phases (Figure 1). Integral to managing the PHE—as an immediate need and acutely practical matter—is the careful and thorough recognition of what each test does and does not measure. For example, some tests are currently restricted to being used only when the user has concurrent COVID-19 symptoms, reducing their applicability in the preventive phase or with those who may be asymptomatic but still disseminating the virus. Determining the role of diagnostics in these 3 phases and how they are then covered by health plans or local authorities should serve as a foundation for sensible polices that provide predictability of benefit in mitigating the PHE and incentives to those creating these critical tools.
Prevention
While social distancing is the primary approach to prevent the spread of the virus to date, more regular diagnostic testing as a part of contact tracing could benefit mitigation efforts. Major barriers exist, including delayed results, strict access requirements (e.g., people who must have symptoms or a doctor’s note to receive a test), and cost-related factors such as major discrepancies in coverage. These challenges could be addressed with policies promoting more streamlined testing strategies and broader coverage. While having a variety of authorized tests is valuable, their appropriate use from a clinical and financial perspective is key to optimal outcomes for individual patients and the US economy.
Infection
While an individual is infected with the virus, testing can be used to guide treatment decisions. A complete understanding of what each test measures, false positive and false negative rates, and the timeliness of the results is important both for treating COVID-19 and for developing clinical guidelines for newly infected patients. In some cases, however, the relationship of treatments to diagnostics is 1:1 based on Food & Drug Administration approval for the treatment, which complicates coverage if providers use a different diagnostic. An example of this is with the recent Emergency Use Authorization for convalescent sera where a specific test and titer is required.
Recovery
In the recovery stage, uncertainty remains as to how long patients experience symptoms and whether they remain contagious. Testing will remain a crucial driver as we pursue a comprehensive understanding of both the short- and long-term impacts of COVID-19. Additionally, as vaccines become available, whether testing to show a lack of exposure would be required for access to a vaccine or testing to demonstrate successful antibody development is uncertain.
Conclusion
As the pandemic progresses and more treatment options become available, testing capabilities will continue to evolve and expand as a crucial piece of the COVID-19 mitigation strategy, both for individuals who contract the virus and the population overall. Diagnostic sponsors should anticipate changes in both the progression of the disease over time and the treatment/vaccine landscape when considering opportunities for development. Additionally, developers should engage with policy and regulatory strategy decision makers to increase access to all types of COVID diagnostics.
To receive Avalere updates, connect with us.
January 23, 11 AM ET
Learn More


