Majority of API in US-Consumed Medicines is Produced in the US
Summary
New analysis of trade data finds that 54% of API, in dollars, used in domestically consumed medicines came from the US in 2019.The US healthcare system relies on the domestic production of active pharmaceutical ingredients (API) supplemented by imports. Avalere’s analysis finds that in 2019, the dollar value of API made in the US accounted for a majority (54%) of the $86.5 billion of API used in medicines consumed in the US. Total dollar value of API made in European Union member states accounted for 26%. Ireland (19%), China (6%), Singapore (3%), the United Kingdom (3%), and Switzerland (2%) are the top 5 individual countries whose API is used in medicines consumed in the US.
Recently, policymakers have focused on the US pharmaceutical supply chain and the role of API that is produced outside of the US and used in US-consumed finished pharmaceutical products (FPP). API used in medicines consumed in the US enter the supply chain in 3 ways: domestically manufactured API, API imported from other countries that is used domestically to produce FPP, and API produced in other countries (non-US manufactured) that is used to produce FPP imported by the US.
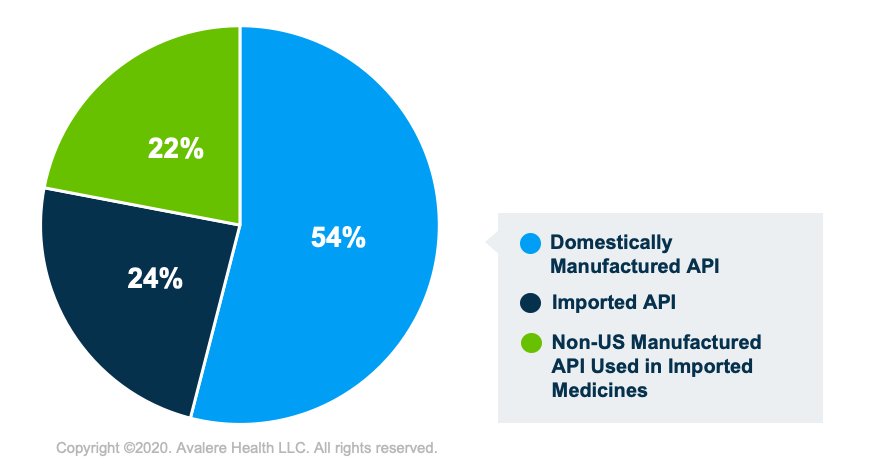
Avalere estimates (Figure 1) that 54% of the $86.5 billion of API used in medicines consumed in the US are produced domestically. Direct imports of API by US manufacturers to domestically produce FPP account for 24% of the API used for medicines consumed in the US. and 22% comprises API that was produced in other countries that is used in FPP imported to the US.1
By summing the total dollar value of API from each of the 3 categories by its country of origin, Avalere was able to estimate the top 15 countries that supply API used in medicines consumed in the US (Table 1).
| Rank | Country | Percent of Total API |
|---|---|---|
| 1 | USA | 54% |
| 2 | Ireland | 19% |
| 3 | China | 6% |
| 4 | Singapore | 3% |
| 5 | United Kingdom | 3% |
| 6 | Switzerland | 2% |
| 7 | Belgium | 2% |
| 8 | India | 2% |
| 9 | Germany | 1% |
| 10 | Italy | 1% |
| 11 | France | 1% |
| 12 | Netherlands | 1% |
| 13 | Spain | 1% |
| 14 | Republic of Korea | <1% |
| 15 | Argentina | <1% |
Overall, Avalere’s analysis found that the API used to produce medicines consumed in the US are both manufactured domestically and imported from other countries. In 2019, the US was the largest supplier of API by cost in medicines consumed in the US—producing nearly 3 times more than the next largest source (Ireland, at 19%). No single foreign country dominates the overall supply of API for the US market.
Funding for this research was provided by Pharmaceutical Research and Manufacturers of American (PhRMA). Avalere retained full editorial control.
To receive Avalere updates, connect with us.
Methodology
Avalere analyzed international trade patterns between the US and other countries. In addition, Avalere analyzed US data on industry output and intermediate purchases to estimate the amount of API in medicines consumed in the US that are made in the US. Domestically manufactured API was calculated by estimating the dollar value of US manufacturers’ intermediate purchases of API and the net of imported API (imported for purposes of US FPP production), plus US exports of API that are then imported to the US in the form of FPP.
Avalere analyzed data from the US Census Bureau’s USA Trade Online for 2019 to determine the top 15 countries, by US dollar value, that export API to the US. To estimate API in US imports of FPP, Avalere used a combination of Census Bureau data and the United Nations Comtrade data. Census Bureau data were used to identify US imports of FPP, both total dollar value and countries of origin, while UN data were used to identify API imports for countries exporting FPP to the US.
For each country exporting FPP to the US, Avalere applied the country composition of their API imports to their exports of FPP. Attribution of imported API dollar amounts to FPP exports to the US was based on the percentage of each country’s FPP exports to the US relative to their total FPP exports. Avalere used US Industry Accounts data from the Bureau of Economic Analysis, US Department of Commerce, to estimate the amount of API purchased by US manufacturers from domestic suppliers.
Avalere identified API in the international trade data by defining it to include harmonized tariff system (HTS) Chapter 29 codes that had an end-use classification of pharmaceuticals (40100). For Census Bureau data, 10-digit HTS codes were used. For UN Comtrade data, 6-digit HTS codes were used. Avalere identified FPP in the international trade data by defining it to include HTS Chapter 30 codes, except for 3005 and 3006, which include bandages and pharmaceutical goods.
Avalere used US dollars to value API for this analysis because that measure, unlike measures like volume by weight, was consistently available across all data sources.
Notes
- The US exports API to many countries that manufacture medicines then imported by the US. Avalere estimates that in 2019 about 2% of the API used in medicines consumed in the US were made in the US and then exported to another country for use in manufacturing FPP that were then consumed in the US.







