TPNIES Adjustment Promotes Access for Innovative ESRD Technologies
Summary
Medicare offers an add-on payment to facilities furnishing qualified new and innovative renal dialysis equipment and supplies via the Transitional Add-on Payment Adjustment for New and Innovative Equipment and Supplies (TPNIES).Given the increased investment in the development of innovative therapies and devices in the dialysis space, manufacturers need to understand the payment pathways that aim to support patient access within Medicare’s bundled payment system. Since January 1, 2011, Medicare has reimbursed facilities via the End-Stage Renal Disease (ESRD) Prospective Payment System (PPS), which is a bundled payment that serves to cover the costs associated with providing most standard dialysis services. In 2016, the Centers for Medicare and Medicaid Services (CMS) introduced a new payment designation known as the Transitional Drug Add-On Payment Adjustment (TDAPA) that allows certain new eligible drugs to receive a temporary separate payment outside of the bundle.
In the 2020 rule-making cycle, the CMS created TPNIES, a similar payment designation for certain eligible new and innovative devices. The intention of this add-on payment is to promote beneficiary access to new and innovative dialysis-related equipment and supplies by providing additional reimbursement to ESRD facilities utilizing such products. The CMS has increasingly sought to promote innovation in the dialysis space with the establishment of the ESRD PPS in 2011, and the TPNIES represents one of the agency’s latest steps in continued pursuit of that goal.
Eligibility Criteria
To receive the add-on payment, applicants for TPNIES are required to demonstrate that the equipment or supply meets the following criteria:
- Is designated as a renal dialysis service by the CMS
- Is within 3 years of Food & Drug Administration (FDA) marketing authorization
- Is commercially available by January 1 of the year in which the payment adjustment would take effect
- Has submitted a complete Healthcare Common Procedure Coding System (HCPCS) Level II code by the application deadline for biannual coding cycle 2 for durable medical equipment, orthotics, prosthetics, and supplies prior to the calendar year
- Has demonstrated evidence of innovation that aligns with substantial clinical improvement (SCI) criteria
- Is not a capital-related asset, except for capital-related assets that are home dialysis machines when used in the home for a single patient.
Unlike the eligibility criteria outlined for TDAPA, the CMS requires evidence of substantial clinical improvement. This is consistent with the agency’s approach for determining eligibility for the New Technology Add-on Payment adjustment under the Inpatient Prospective Payment System. While the CMS does not require that applicants meet explicit targets or thresholds to demonstrate SCI, it provides guidance about circumstances that may allow an applicant’s equipment or supply to qualify. Primarily, the CMS requires that the device provide an improved treatment option for specific patient populations or significantly advance treatment efficacy, disease resolution, or patient health outcomes compared to the standard of care.
Stakeholders should closely monitor changing reimbursement dynamics for eligible technologies. Currently, the CMS will adjust payment based on 65% of the price established by Medicare Administrative Contractors using invoices and other related payment information. The CMS will make this positive payment adjustment for 2 calendar years, after which the device will be considered eligible as an outlier service with no change made to the ESRD PPS base rate. Capital-related assets used as home dialysis machines will not be an eligible outlier service upon expiration of the add-on payment.
Existing Applications and Future Outlook
The CMS received 2 complete applications for the TPNIES designation in the 2021 application cycle. Although neither received the add-on payment designation, both stakeholders are eligible for resubmission in the calendar year 2022 and 2023 application cycles. Given the novelty of this payment designation and the opportunity to improve patient care in this space, stakeholders should closely monitor policy developments or determinations from CMS.
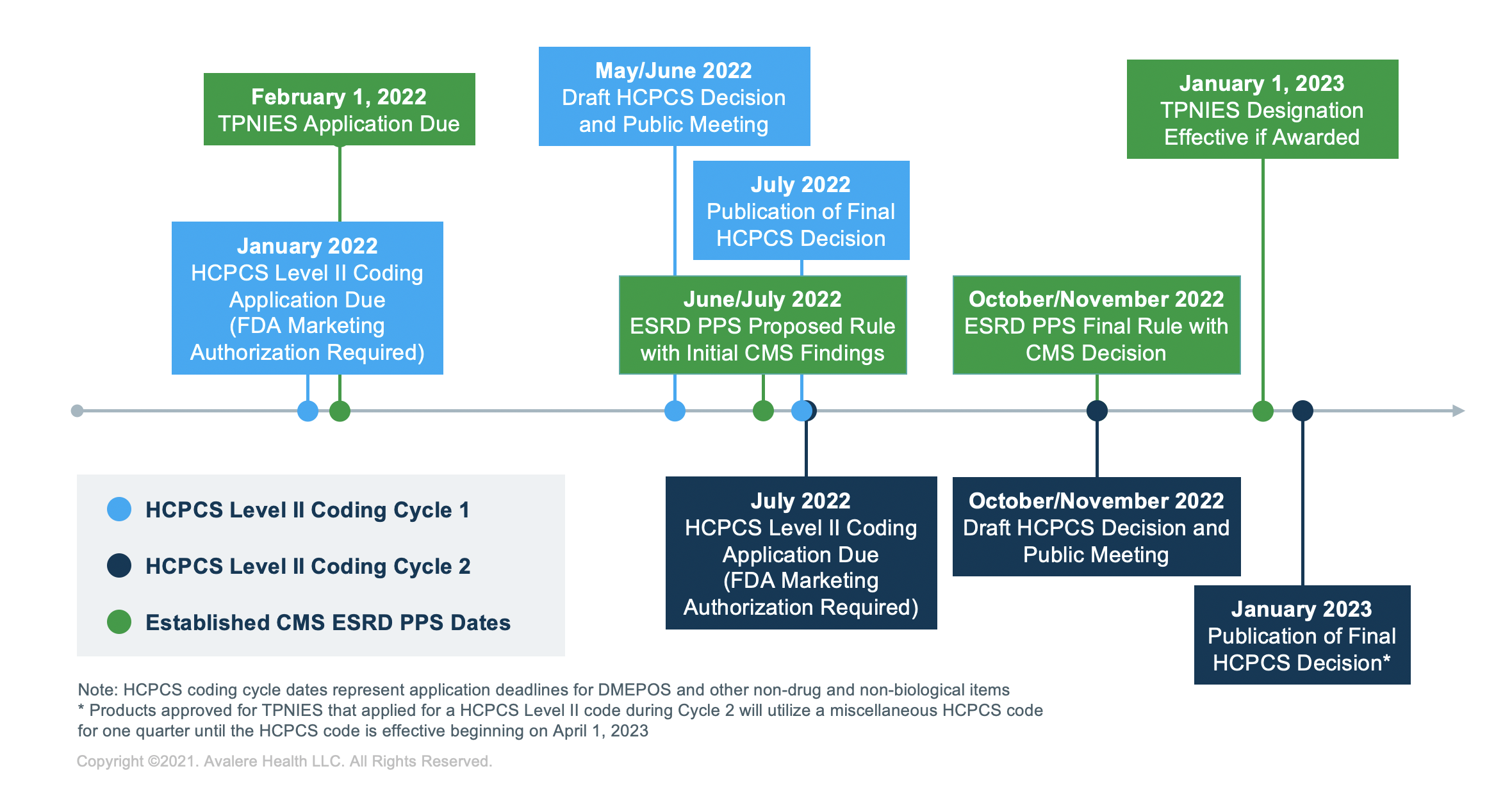
TPNIES Timeline
For stakeholders planning to apply to receive the TPNIES designation in future years, February 1 is the final date to submit a completed application to the CMS to be considered during next year’s rulemaking cycle. Stakeholders must also have FDA marketing authorization and a submitted HCPCS application for the device by the time they apply for TPNIES.
Avalere has deep expertise with the ESRD PPS, as well as the broader kidney care payment and delivery space. Further, Avalere has a proven history assisting clients with applications for additional payment designations and HCPCS codes, and can help stakeholders understand the TPNIES and any changes it may undergo in future rulemaking cycles.
To learn more about how Avalere can support you in understanding the evolving ESRD and kidney care policy and market landscape, connect with us.







