Social Determinants May Affect Access to CAR-T Treatment
Summary
Analysis highlights potential access barriers to a broad set of CAR-T therapiesChimeric antigen receptor T-cell (CAR-T) treatments first came to market in 2017, and since then there has been rapid development and advancement bringing these innovative treatments to a broader set of patients. As of the mid-2024, there are six CAR-T treatments targeting blood cancers. However, the promise of CAR-T has been accompanied by challenges for patient access due to a range of factors, including a limited set of treatment sites, an uncertain reimbursement landscape, a complex supply chain, an extended period of follow-up care, and financial costs that extend beyond drug costs. These unique access barriers for CAR-T must be considered through a health equity lens to better understand the appropriate solutions that should be pursued to bolster access for eligible patients.
Avalere is building on prior research to analyze treatment barriers for patients diagnosed with a broad set of five cancer types that may be treated using on-market CAR-T treatments. The five indications are diffuse large b-cell lymphoma (DLBCL), acute lymphoblastic leukemia (ALL), multiple myeloma (MM), follicular lymphoma (FL), and mantle cell lymphoma (MCL).
In this analysis, Medicare fee-for-service (FFS) beneficiaries with relevant diagnoses were subdivided into distance-based cohorts determined by driving time from home ZIP codes to CAR-T treatment sites. Avalere assessed demographic and socioeconomic factors to understand differences across cohorts that may influence access to care and/or require new approaches to support access that are not based solely on distance.
Avalere found persistent trends across all five conditions showing that patients living nearest to a treatment site were disproportionately likely to be Black or Hispanic compared to the patient population further from a treatment site. Additionally, patients living in areas nearest to treatment sites had lower levels of vehicle ownership and fewer adults per household. Income data showed differences between earnings for White individuals and Black or Hispanic individuals, demonstrating that “need” cannot be easily defined based on proximity to a treatment site and therefore novel forms of support could be necessary.
Background
Treatment with CAR-T products is currently provided in a subset of hospitals with the experience and training necessary to ensure safe and effective care, including management of any potential adverse events. In this analysis, a subset of Foundation for the Accreditation of Cellular Therapy (FACT)-accredited sites that provide immune effector cell therapy were considered as potential CAR-T treatment locations. Policy and regulatory attention has generally been focused on alleviating access barriers for eligible rural patients who may need to travel significant distances, since many FACT-accredited sites are in urban areas with large hospital systems or academic medical centers. However, other factors may impact access for the populations of potential CAR-T patients living close to a treatment center in urban or suburban communities.
Analysis Findings
The defined patient cohort in the analysis was enrolled in Medicare FFS between 2018 and 2021. There were no restrictions applied to the cohort based on the line of therapy or disease progression. Therefore, the analysis captures all DLBCL, ALL, MM, FL, and MCL patients, including those who may not be currently eligible for CAR-T treatment.
Given the rapid development in the CAR-T market and the progression to manage disease as an earlier line of therapy, reviewing a broader set of patients who may present as eligible for future treatment was warranted. This analysis focused on individual- and community-level factors(race/ethnicity, income, household size/composition, and vehicle ownership to) better understand potential needs for patients based on how far they live from treatment sites. This assessment was undertaken with the goal of identifying compounding factors (e.g., additional to drive time to a treatment site) that could contribute to access barriers.
Key findings include:
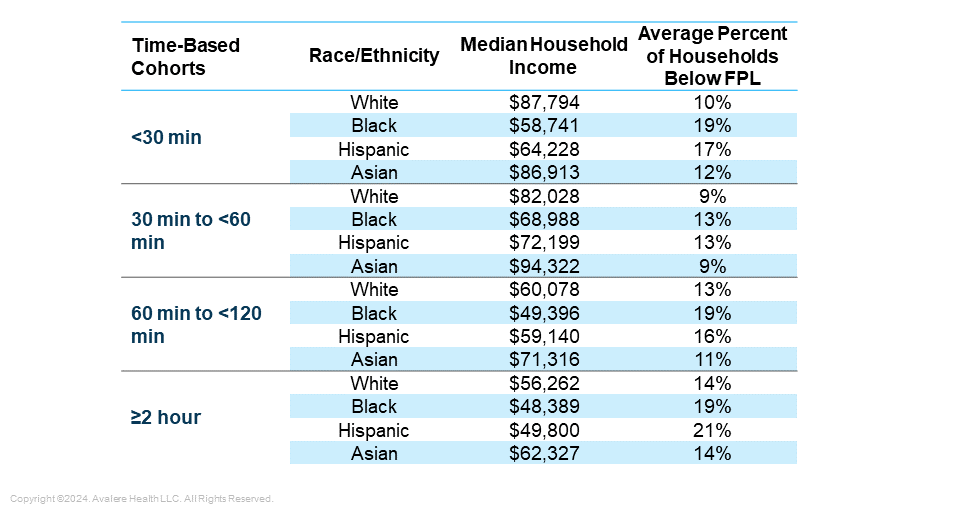
Differences in Indications by Proximity to Treatment Center: Proximity to treatment centers was consistent across all five indications, with the greatest variations in the <30 minute range and the 60–120 minute range. Notably, more than 50% of patients in every indication live within one hour of a cell therapy treatment site (Figure 1).
Figure 1. Potentially Eligible Patient Breakdown Per Cancer Type by Distance to Nearest Treatment Center (2018–2021)
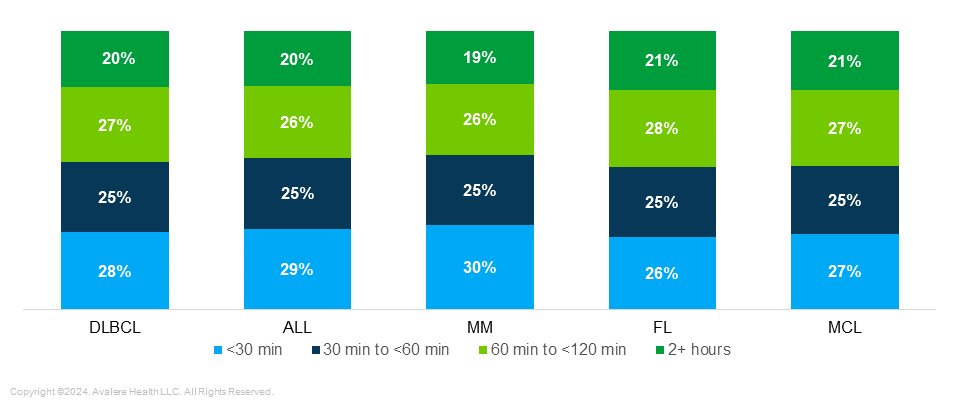
Differences in Race/Ethnicity by Proximity to Treatment Center: When comparing patients across indications, there was a higher percentage of non-White patients diagnosed with MM and ALL, particularly in the cohort less than 30 minutes from a treatment site (Figure 2).
Consolidating all the patients from the five indications and focusing breakdown of race across the distance-based cohorts, highlights the difference in patient populations living within 30 minutes of treatment centers and patients living greater than 60 minutes from a treatment center. Within 30 minutes of a treatment site, 15% of patients are Black and 73% of patients are White compared to the cohort greater than two hours from a treatment site, where 6% of patients are Black and 88% of patients are White. This shows that, proportionally, a greater percentage of Black patients are closer to a treatment site while a greater percentage of White patients are in rural areas.
Nearly half (43%) of patients who are Black and 46% of patients who are Hispanic live within 30 minutes of a treatment site compared to only 25% of patients who are White.
Figure 2. Potentially Eligible Patient Breakdown Per Cancer Type by Race (2018–2021)
Differences in Caregiver Availability: In an assessment of household characteristics, Avalere found that potential CAR-T patients living nearest a treatment site were from areas with a higher share of single householders (44%) compared to those who live more than 30 minutes from a treatment site (38–39%), potentially indicating lower availability of caregiver support in the home, which could be integral during the pre- and post-operative periods around CAR-T treatment.
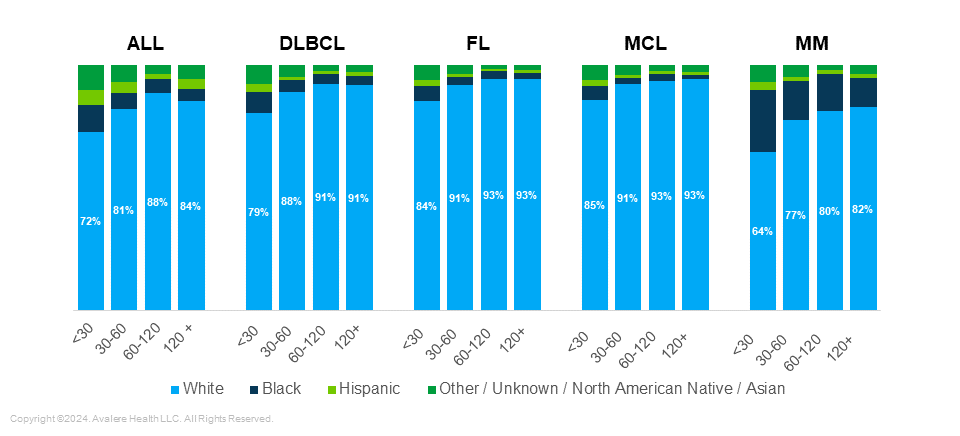
Transportation Options: Compared to patients living more than 30 minutes from a treatment site, those living in closer proximity were from areas with lower levels of vehicle ownership (10–12% of households with no vehicle across all five indications) (Figure 3).
Figure 3. Households without Vehicles by Cancer Type and Distance to Nearest Treatment Center (2018–2021)
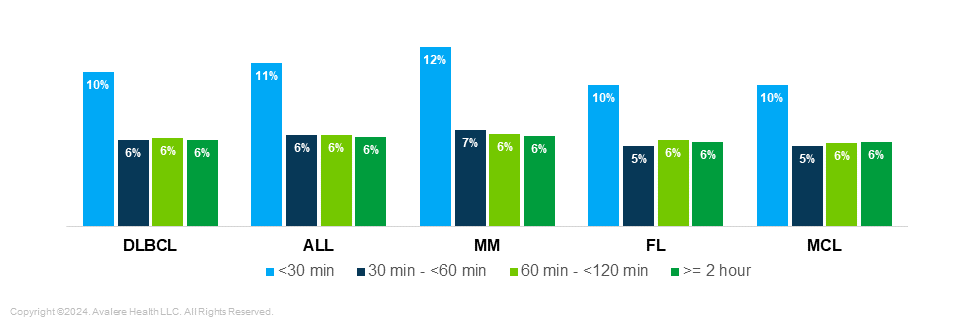
Differences in Income: Black and Hispanic patients living less than 30 minutes from a treatment site were from areas with lower median household incomes than White patients in the same cohort, indicating potential cost barriers and higher need among minority patients close to treatment centers (Figure 4) . Median household income for Black patients within 30 minutes of a treatment site was $58,741, and Hispanic patients earned $64,228, compared to $87,794 for White patients. In general, incomes for Black and Hispanic patients were lower than those of White patients in all distance cohorts.
Figure 4. Income of Cohorts by Race/Ethnicity (2018–2021)
Key Considerations for Patient Support and Policy
The findings of this analysis build on prior observations related to access in the CAR-T space, with implications across stakeholders:
- The findings highlight the need to approach CAR-T access in a holistic manner that accounts for barriers other than distance from a treatment site. Additionally, solutions pursued with a goal of improving access should be evaluated for unintended consequences or potential for creating/exacerbating disparities. For example, among patients living near a treatment site, there may be an acute need for wraparound services (e.g., rides to treatment centers, temporary on- or near-campus housing at treating sites, respite for caregivers, health aide assignment in absence of family caregivers, meal delivery for low-income patients while in treatment). These services could be coordinated and provided by a combination of treating sites, health plans, manufacturers, and other organizations that serve patients.
- There are a range of factors that may impact uptake of a CAR-T treatment, including provider reimbursement, out-of-state travel for treatment, and manufacturing capacity/supply chain. This research shows that even among populations viewed as facing fewer barriers to treatment due to their relative proximity to treatment locations, access to CAR-T can be impeded by other factors such as affordability, transportation options, and lack of caregiver support. Successful patient access strategies are likely to be those that provide patients with multiple options for support and incorporate considerations related to patient age, language, race and ethnicity, and income.
- Policymakers should consider whether current regulatory barriers limit support options for patients living at different distances from treatment centers and assess the impact of amending existing policies, or creating exemptions, to allow stakeholders to more effectively address social determinants of health and non-physical barriers to accessing care.
Methodology
Using 2018–2021 data, Avalere identified a population of previously treated MM patients. Medicare FFS beneficiaries were identified using the 100% file of Medicare FFS Parts A and B data, accessed via a research-focused data use agreement with the Centers for Medicare & Medicaid Services; commercial, Medicare Advantage, and Medicaid managed care members were identified using medical claims from the Inovalon MORE2 Registry®, a large scale, real-world multi-payer dataset comprising medical, pharmacy, and lab claims, as well as clinical data on more than 332 million de-identified patients. Patients were identified with one of following five indications: acute lymphoblastic leukemia, diffuse Large B-cell lymphoma, follicular lymphoma, mantle cell lymphoma, and multiple myeloma.
Identified ALL, DLBCL, FL, MCL, and MM, patients were placed into distance-based cohorts using average drive time calculations to the closest prospective treatment site. Avalere leveraged the Acxiom’s InfoBase® Geo files database and the American Community Survey to assess socioeconomic factors in the relevant geographic areas in which each individual resides.
Funding for research provided by PhRMA. Avalere Health retained full editorial control.
January 23, 11 AM ET
Learn More