Socioeconomic Factors May Impact Patient Access to Cell Therapies
Summary
Analysis finds that patients face barriers to cell therapy treatments regardless of proximity to cell therapy treatment sites.Patients requiring treatment with innovative cell and gene therapies can access them at a limited number of authorized treatment centers (ATCs) and often require support before, during, and following treatment. Some patients may need to travel significant distances to access an authorized site (sometimes across state lines), and patients may face other barriers associated with financial resources, caregiver support, transportation, or lost wages. In a previous analysis, Avalere focused on the geographic barriers to treatment, highlighting the median average distance patients must travel to a treatment site by census region and the potential need for patient support services that take into account treatment costs, patient travel, non-drug expenses, long-term follow-up, and care coordination.
A new Avalere analysis of patients with multiple myeloma (MM) focuses on barriers to treatment beyond geographic location. In this analysis, potentially eligible Medicare Fee-for-Service (FFS) patients were subdivided into distance-based cohorts based on their driving time to cell therapy treatment sites. Avalere assessed demographic factors and other socioeconomic characteristics between groups. Avalere found that certain patients who live near a treatment site and are potentially eligible for cell therapy may face barriers to obtaining novel treatment despite their relative proximity to the center. Individuals living nearest to treatment sites were disproportionately Black and Hispanic compared to those living more than 2 hours from a site and were from areas with fewer adults in the household, more single householders, and lower vehicle ownership, which could impact access and outcomes.
Background
Given the requirement for monitoring patients following administration of a chimeric antigen receptor T-cell (CAR-T) product, cell therapies must be administered at highly regulated ATCs. ATCs represent a limited subset of medical centers that typically receive both manufacturer certification and third-party accreditation (e.g., Foundation for the Accreditation of Cellular Therapy accreditation), requiring providers to meet specific training requirements and have the ability to safely manage adverse events. These sites must also satisfy Food & Drug Administration risk evaluation and mitigation strategy requirements. Many ATCs are currently located in urban areas within large hospital systems or academic medical centers. Some eligible patients may need to travel significant distances to access care, but sizeable populations of eligible patients also live in urban or suburban communities with relatively shorter distances to travel for treatment. While emphasis has been placed on solving access issues for rural patients, other factors may impact access for those living close to a treatment center.
Analysis Findings
The analysis focused on previously treated MM patients enrolled in Medicare FFS between 2014 and 2019. Avalere analyzed demographic information for these patients living at various driving distances from potential cell therapy ATCs. Additionally, Avalere assessed socioeconomic factors for the locality where each patient resided. This assessment was undertaken with the goal of identifying factors beyond driving time to a treatment site that could contribute to access barriers and may need to be addressed to ensure equitable access. Key findings include:
- Differences in Race/Ethnicity by Proximity to Treatment Center: When comparing previously treated MM patients living within 30 minutes of a treatment site and those living more than 2 hours from a site, a larger share of non-White populations (approximately 40% of Black and Hispanic patients) were in the cohort living nearest the treatment site compared to White patients (22%). Given well-documented associations between race/ethnicity and the risk of poor health outcomes, limited earning potential, and other associated impacts, it is important to take into account the unique barriers to care and needs for patients of color.1,2,3
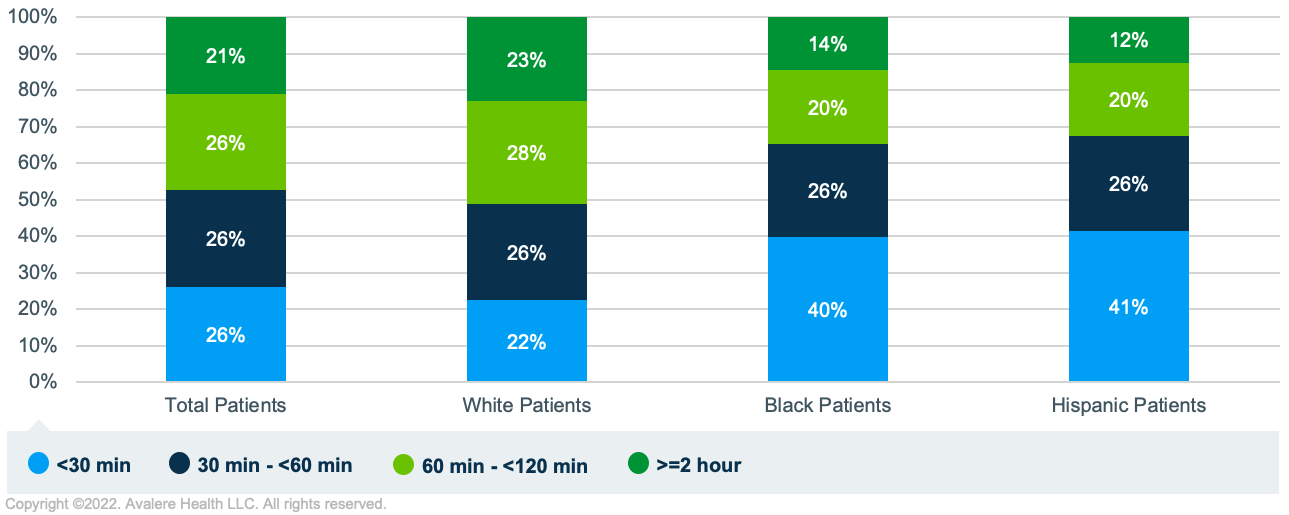
- Differences in Caregiver Availability: Many MM patients accessing cell therapy will require an extended stay near a treatment site and would benefit from a caregiver to assist with transportation and support afterwards. In an assessment of household characteristics, Avalere found that among previously treated MM patients living nearest a treatment site, there were a higher share of single householders, fewer adults per household, and lower marriage rates, potentially indicating less caregiver support in the home.
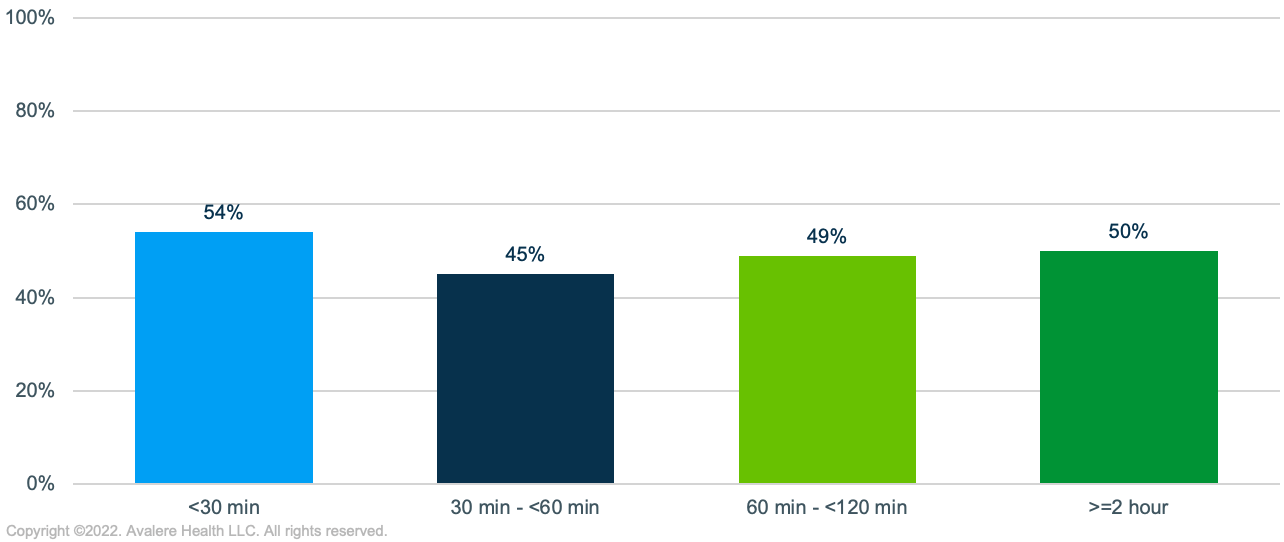
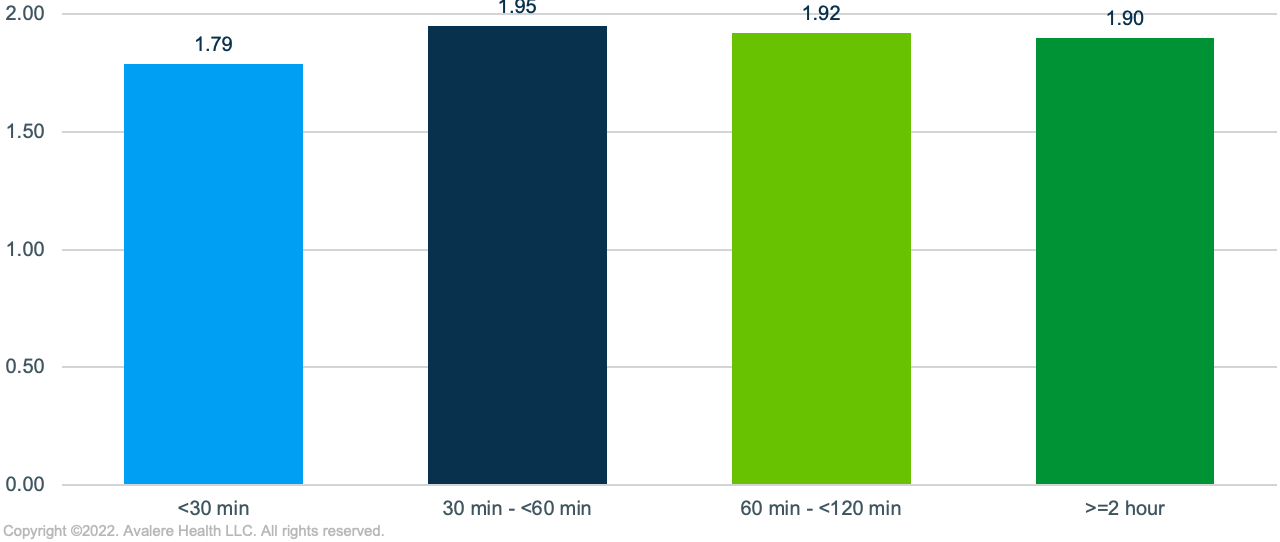
- Transportation Options: Compared to previously treated MM patients living more than 2 hours from a treatment site, those living within closer proximity of a site were from areas with lower levels of vehicle ownership (13% of households with no vehicle). Conversely, individuals living more than 2 hours away were more likely to own 2 or more vehicles, and thus have multiple options for traveling to access treatment. While individuals living close to ATCs may have access to public transportation or ride-sharing options, these may not be preferred modes of transport following treatment or could become a cost burden over the course of pre- and post-treatment visits. Additionally, significant variance exists in the comprehensiveness and reliability of public transportation options by city.
- Differences in Income: Black and Hispanic MM patients living in a 30-minute radius were found to reside in areas with lower median household income than White patients living at a similar distance, indicating a high need particularly among minority patients that are close to treatment centers. When adjusting for cost of living, Black patients’ median household income in this distance range was $48,231 and Hispanic patients’ was $43,387, compared to $71,084 for White patients. Incomes for Black and Hispanic patients in these areas were also lower than those of White patients living more than 2 hours from a treatment site.
Key Considerations for Patient Support and Policy
- These findings highlight the importance of considering factors beyond driving time or distance from a treatment site when crafting patient support offerings for cell and gene therapies. Access to cell therapy can be impeded by other factors such as affordability, transportation options, and lack of caregiver support. Successful patient support strategies are likely to be those that provide patients with multiple options for support and incorporate considerations related to factors such as patient age, language, race and ethnicity, and income status.
- Given that current patient support services offered by ATCs and patient organizations are limited and have been subject to significant strain during the COVID-19 pandemic, future investments in new and more comprehensive support services may be necessary as the demand for CAR-T therapy increases and as more cell and gene therapies are brought to market. Specifically, policymakers should consider whether current regulatory barriers limit support options for patients living at different distances from treatment centers and assess the impact of amending existing policies or creating exemptions to better allow stakeholders to address social determinants of health and non-physical barriers to accessing care.
- Policies affecting access to treatment and patient support could also be assessed to determine whether the policy would unintentionally contribute to racial or socioeconomic inequities in access to care.
Funding for research provided by Janssen Pharmaceuticals. Avalere Health retained full editorial control.
To receive Avalere updates, connect with us.
Methodology
Using data from 2014–2019, Avalere identified a population of previously treated MM patients. FFS beneficiaries were identified using the 100% file of Medicare FFS Parts A and B data, accessed via a research-focused data use agreement with the Centers for Medicare & Medicaid Services, and commercial, Medicare Advantage, and Medicaid managed-care members were identified using medical claims from the Inovalon MORE2 Registry®, a large scale, real-world multi-payer dataset comprising medical, pharmacy, and lab claims, as well as clinical data on more than 332 million de-identified patients.
Identified MM patients were placed into distance-based cohorts using average drive-time calculations to the closest prospective treatment site. Avalere leveraged the Acxiom database to assess socioeconomic factors in the relevant geographic areas in which each individual resides.
Notes
- Centers for Disease Control, “Health Equity Considerations and Racial and Ethnic Minority Groups,” April 2021.
- Agency for Healthcare Research and Quality, “Priority Populations: Racial/Ethnic Minorities,” June 2021.
- Williams, D., et al. “Understanding Associations between Race, Socioeconomic Status, and Health: Patterns and Prospects,” Health Psychology, Division of Health Psychology, American Psychological Association, 35(4), 407–411.




