 Mapping a Complex Drug Launch Strategy
Mapping a Complex Drug Launch Strategy
Summary
Preparing to bring an innovative oncology drug to market, a clinical-stage therapeutics company sought our assistance in developing a multifaceted launch strategy. With our support, the client met its goals and received Food & Drug Administration fast-track drug designation.Client Type
A privately held cancer therapeutics company seeking to transform care for hard-to-treat cancers.
Challenge
Prior to launching its new cancer therapy, the client lacked experience in drug pricing and launching within a specific channel. The new drug had multiple uses, further complicating the launch. The client solicited our advice on which of its indications to highlight, as the current reimbursement system poses challenges for multi-indication treatments.
Solution
We conducted a thorough assessment of key pricing and access drivers for multi-indication cancer therapies. The assessment, which included a wide array of factors to determine the best price range for the new drug, provided background knowledge on traditional oncology pricing and reimbursement considerations.
We used our market access analytics capabilities to model various pricing scenarios in order to understand opportunities and risks for access and utilization. Using these insights, we helped the client gauge the benefits and drawbacks of each pricing scenario. The data that informed this process will continue to be useful in shaping future plans for the product.
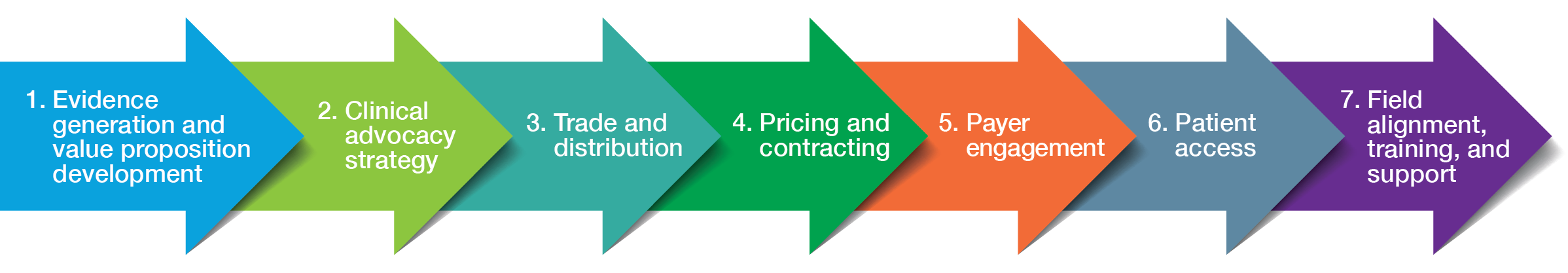
Using these data, we created a roadmap tailored to the product’s intended patient population and its unique opportunities and challenges. The roadmap concentrated on key areas for a successful commercial launch, including payer engagement, channel/distribution, and patient access. It included comprehensive recommendations that detailed customer engagement and access, marketplace trends, and anticipated requirements. After exploring the roadmap in depth with the drug company, we developed a tactical market-launch playbook.
Outcome
Guided by our insights, the client reached its milestones quickly and was able to track progress efficiently during the drug launch process and monitor subsequent drug enrollment.
Engage An Avalere Expert Today.



