Affordable Patient Access to PCSK9 Inhibitors Remains Challenging Across Part D Plans in 2020
Summary
Avalere analysis finds that, despite substantial list price decreases across the PCSK9 inhibitor class, out-of-pocket costs for the majority of 2020 Part D beneficiaries remain significantImproving affordability for prescription drugs, especially for seniors, has remained a key priority in Washington for the past year, both on Capitol Hill and with the administration. High out-of-pocket (OOP) exposure for patients, particularly for those with chronic conditions, can threaten access and adherence and may lead to worsened patient outcomes.
PCSK9 inhibitors, a relatively new class of drugs designed to lower cholesterol levels and reduce the risk of heart attacks and strokes, have encountered challenges with uptake. When added to traditional statin therapy for patients who do not respond adequately to statins, these drugs can reduce the risk of cardiovascular events, but payers have been restricting access through tight utilization management and restrictive formulary placement in part due to concerns over cost and appropriate use.
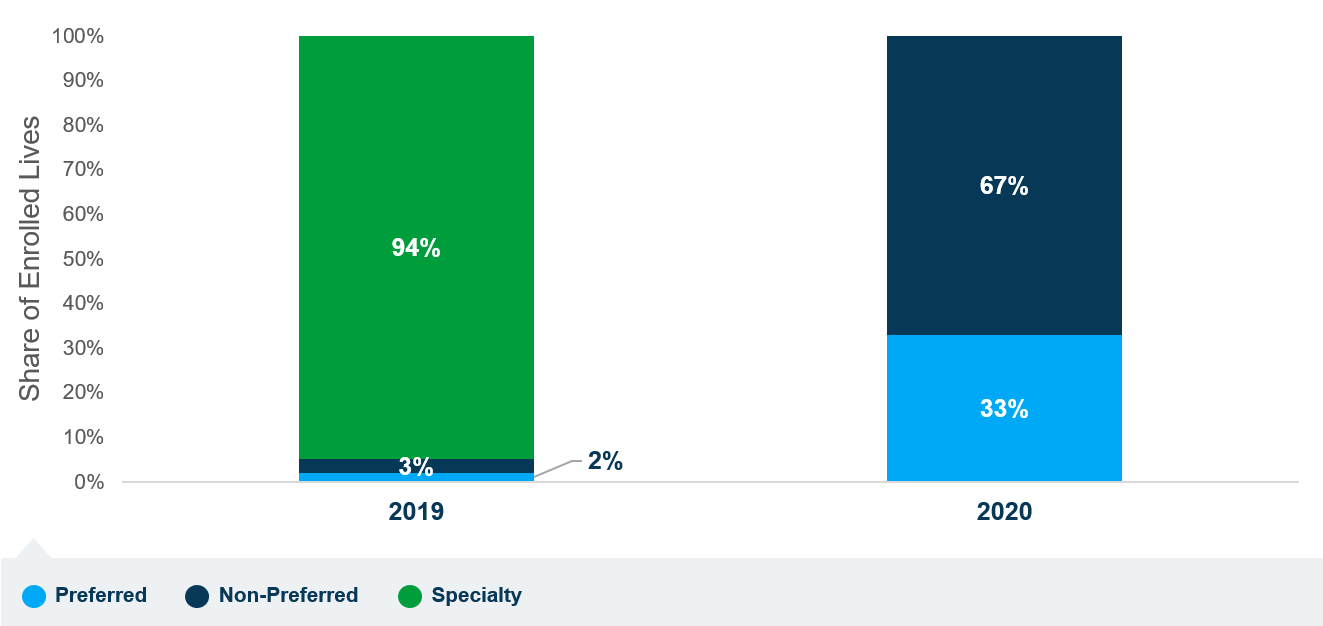
In an effort to alleviate affordability concerns, starting in late 2018, one manufacturer of PCSK9 inhibitors made these products available at a 60% reduction to the original list price, and another manufacturer followed in 2019. CMS Administrator Seema Verma, in a public letter to members of Congress in August, recognized that PCSK9 inhibitor prices dropped below the Part D specialty tier cost threshold ($670 per month in 2020), which she believed would allow Medicare beneficiaries to access the products on lower-cost tiers. However, a recent Avalere analysis of 2020 Part D formularies finds that, while all plans have removed PCSK9 inhibitors from the specialty tier, only a third of all Medicare Part D beneficiaries will be able to access those products on preferred brand tiers in 2020. Instead, most Part D plans have shifted PCSK9 inhibitors to non-preferred tiers, which can be associated with higher OOP costs than the specialty tier.
Figure 1. Tiering of PCSK9 Inhibitors Across All Part D Plans (2019–2020)
OOP costs for Medicare patients are determined by many factors, such as income, health status, type of coverage, formulary design, and price of prescribed therapies. As a result, a patient’s OOP costs for PCSK9 inhibitors can vary substantially across the Part D program. CMS caps coinsurance for Part D plan specialty tiers between 25% and 33%, but cost sharing for non-preferred drug tiers can be as high as 50%. Indeed, Avalere’s analysis finds that, in 2020, the majority of patients without low income subsidies (LIS) under Part D will continue to face high monthly costs. In 2020, almost 60% of Part D beneficiaries will face cost sharing of $100 per month or more to access PCSK9 therapies.
Figure 2. Distribution of Non-LIS Patient OOP Costs for PCSK9 Inhibitors, 2020
Moreover, regional differences in plan offerings might result in disparities of access and affordability across the US. As required under the Medicare Modernization Act, Part D plans operate around geographic regions established by CMS, with different premiums and coverage. Avalere analysis finds that more than 80% of Part D beneficiaries in areas of the Northeast US and California can access PCSK9 inhibitors only on non-preferred tiers in 2020, which could impact their OOP liability.
Figure 3. Percent of Part D Lives by State in 2020 with Access to PCSK9 Inhibitors Only on Non-Preferred Tiers
CMS reviews Medicare prescription drug plan formularies to ensure that beneficiaries have access to clinically appropriate medications and that plans do not discriminate against certain enrollees. The agency’s guidance to Part D plan sponsors indicates that best practices in formulary management generally place drugs in a less preferable position only when drugs that are therapeutically similar (i.e., drugs that provide similar treatment outcomes) are in more preferable positions on the formulary. Despite this guidance, Avalere’s analysis suggests that, in 2020, only one third of Part D beneficiaries will have access to a preferred option across the PCSK9 inhibitors class, despite recent decreases in list price.
Funding for this research was provided by Amgen. Avalere retained editorial control.
Methodology
Avalere analysis of PCSK9 therapies access relied primarily on Part D formulary and health plan benefits data obtained from CMS. First, Avalere estimated the share of all Part D lives in 2019 and 2020 that would be able to access a PCSK9 therapies—either Repatha or Praluent—at each tier level. For every Part D plan, the analysis identified the lowest tier placement for PCSK9 therapies and in cases when both are covered on the formulary, Avalere picked the product on a lower tier. Avalere then aggregated this information across all Part D plans and weighted by September 2019 enrollment to determine the share of Part D covered lives that would have access to PCKS9s at each tier level. Avalere notes that weighting 2020 plans by September 2019 enrollment will minimize the impact of new plans in 2020. Due to data availability (i.e., 2020 enrollment will not be available until February 2020), Avalere applied this method to ensure results reflect patient rather than plan shares.
Next, the analysis estimated non-LIS patient OOP costs for filling a single PCSK9 therapies script in 2020. Avalere excluded the LIS population, because they pay flat low copay amounts that are set annually through CMS rulemaking and do not vary by brand. Avalere assessed the tier-specific cost-sharing amount for each covered PCSK9 therapy. In the case of a coinsurance, Avalere multiplied the product’s wholesaler acquisition cost by the patient’s liability percentage. In the case of plans with multiple covered drugs, Avalere included the product with the lowest OOP cost. Those results were then aggregated across all plans and enrollment-weighted to determine the share of patients who would have to pay more than $100 to access their PCSK9 therapies.
Finally, Avalere’s state analysis determined the share of all Part D lives in each state who only have access to PCSK9 therapies on the non-preferred tier. Similar to the overall tier distribution, Avalere assessed the tier placement of PCSK9 therapies and counted plans in which all covered PCSK9 therapies were on the non-preferred tier. Note that as of 2020, all covered PCSK9 therapies were on the preferred or non-preferred tiers. Avalere then merged the results with state-specific plan enrollment and aggregated the share of lives to generate state-specific estimates.
To receive Avalere updates, connect with us.
Find out the top 2020 healthcare trends to watch.
Webinar | Election 2024: What's at Stake for Healthcare?
On August 14 at 1:30 PM ET, Avalere experts and guests will discuss the 2024 elections, exploring the candidates’ health policy approaches and implications for stakeholders.