CAR-T Reimbursement Continues to Evolve in FY 2023 IPPS Proposed Rule
Summary
Policy proposals for Medicare’s CAR-T inpatient reimbursement build on market developments as bundled payment incorporates additional treatments.In the fiscal year (FY) 2023 Inpatient Prospective Payment System (IPPS) proposed rule, the Centers for Medicare & Medicaid Services (CMS) proposed continuing to use its Medicare Severity Diagnosis-Related Group (MS-DRG) for Chimeric Antigen Receptor T-cell (CAR-T) treatment stays, with differential reimbursement based on whether the product was provided as part of a clinical trial. This year, the CMS proposed a return to using recent spending data (from 2021) to establish the relative weight for the MS-DRG after skipping use of 2020 data, which was impacted by the COVID-19 pandemic’s effects on inpatient utilization patterns. The CMS did not propose adding any additional procedure codes affecting pre-Major Diagnostic Category MS-DRG 018, “Chimeric Antigen Receptor (CAR) T-cell and Other Immunotherapies,” though utilization of 2021 data will result in reimbursement incorporating additional treatments and cases. The financial impact of these changes will vary by hospital, and reimbursement may continue to fall short of fully recognizing provider costs of treatment in some cases.
Background
Since the first Food & Drug Administration (FDA) approval of a CAR-T product in 2017, concerns have persisted over how the Medicare program would reimburse for these products, which are currently administered in the inpatient setting and have a significant cost for providers (e.g., $373,000 average sales price for 1 indication). Hospital inpatient reimbursement is calculated on an episodic basis using a MS-DRG base payment rate that is adjusted for factors such as hospital geography, new technology add-on payment (NTAP), and outlier payments.
In FY 2022, inpatient stays with CAR-T treatment are assigned to DRG 018 (Chimeric Antigen Receptor (CAR) T-cell and Other Immunotherapies), which has a base reimbursement rate of $246,955. Hospitals may receive additional payments for 2 products with NTAP status (TecartusTM and Abecma®); however, the NTAP is limited to 65% of the product cost. Outlier payments are available to hospitals to cover extremely costly cases in which the costs exceed the MS-DRG payment, the NTAP amount (if applicable), and the fixed-loss threshold of $30,988. Even with these adjustments, Medicare reimbursement for CAR-T cases today sometimes fails to cover total hospital costs, with potential negative impacts on provider uptake and patient access.
Proposed FY 2023 Changes
For FY 2023, the CMS proposed several policies that would impact provider reimbursement for CAR-T.
Payment Changes for CAR-T Cases
As a result of an increase to the proposed base operating and capital rates for all IPPS payments and a slight decrease in the proposed relative weight for MS-DRG 018, the proposed base payment for CAR-T cases in FY 2023 would increase by 0.8% to $248,806. However, the CMS proposed increases to the fixed-loss amount, which raises the threshold for outlier payments to be issued. The CMS proposed to increase the fixed-loss amount from $30,988 to $43,214 (a 39% increase) in FY 2023 using a methodology leveraging pre-public health emergency inflation estimates. If the CMS were to use an alternative approach incorporating public health emergency-era data, the fixed-loss amount would be $58,798 (a 90% increase). The CMS requested comment from stakeholders on which approach to use for FY 2023. For CAR-T cases, which are more likely than other inpatient stays to qualify for outlier payments, this could lead to lower overall reimbursement in FY 2023, as illustrated in Figure 1 below.
Use of 2021 Data to Establish Payment
The CMS proposed using 2021 Medicare Provider Analysis and Review (MedPAR) data to set relative weights for MS-DRGs. Additionally, the CMS proposed setting relative weights by first calculating the weights with all COVID-19 stays included in the data, then calculating relative weights excluding COVID-19 stay, and averaging the 2. The use of more recent data means that the hospital charge data incorporates utilization of 3 additional products for non-clinical trial cases—TecartusTM, BreyanziTM, and Abecma®—that were not FDA approved in 2019, which previously formed the basis of FY 2022 reimbursement calculations.
Adjustment for Clinical Trial Cases
The CMS proposed continuing to reimburse for CAR-T clinical trial cases, which do not incur drug costs, at a lower rate than non-clinical trial cases. The CMS stated in the FY 2023 proposed rule that, based on a review of 2021 MedPAR data, clinical trial cases for CAR-T treatment typically cost 20% of non-clinical trial cases and therefore proposed applying an adjustment factor of 0.20 to the relative weight of MS-DRG 018 for these cases. If finalized, this would be an increase over the current adjuster of 0.17 and would result in a base rate for clinical trial cases of $49,761 in FY 2023, a 19% increase over FY 2022.
Product NTAP Decisions
Two CAR-T therapies with NTAP status for FY 2022 will continue in FY 2023:
- Abecma® (idecabtagene vicleucel), for treatment of relapsed/refractory multiple myeloma
- TecartusTM (brexucabtagene autoleucel), for treatment of relapsed/refractory mantle cell lymphoma
The CMS will accept public comment on whether the following CAR-T product meets the newness, cost, and clinical improvement criteria required for NTAP status in FY 2023:
- CarvyktiTM (ciltacabtagene autoleucel), for treatment of relapsed/refractory multiple myeloma
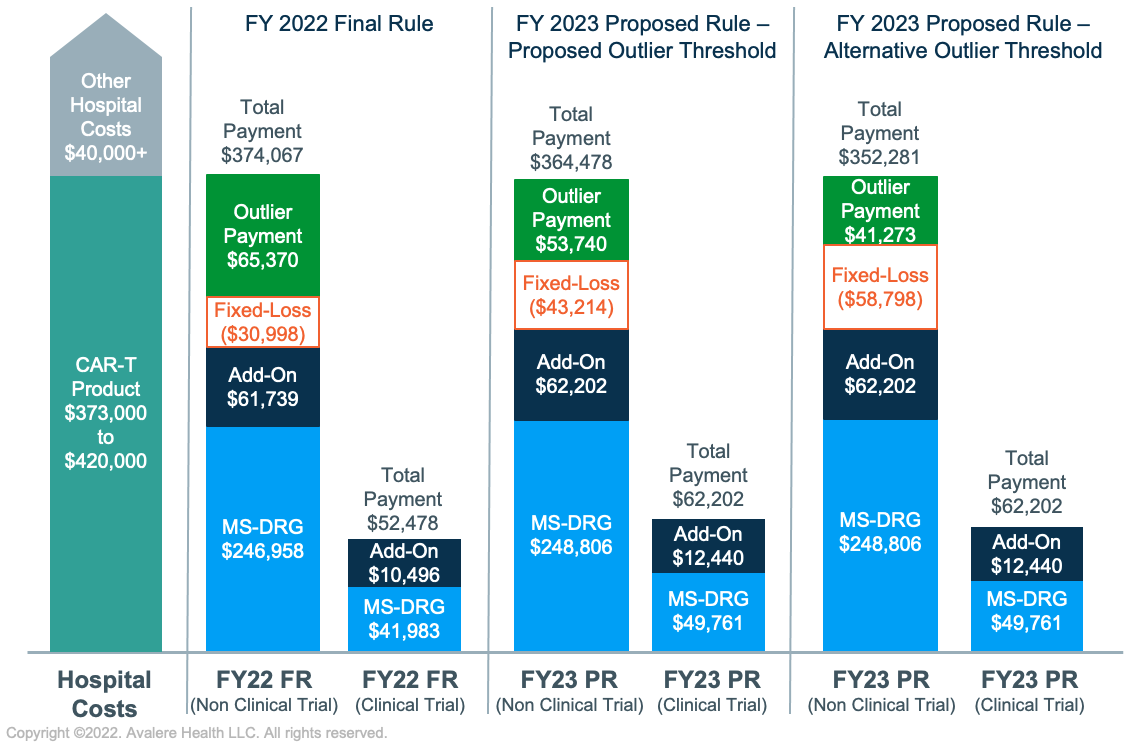
Figures not to scale.
Assumptions:
- Hospital charges for CAR-T episode are kept constant across all examples, consistent with the geometric mean charges included in the FY 2023 Final Rule AOR/BOR file ($1,404,657)
- Hospital has an average operating and capital cost-to-charge ratio of 0.3
- Hospital has an indirect medical education adjustment factor of 0.2 and disproportionate share hospital adjustment of 0.05
- Hospital area wage index is 1.0
Key Considerations Looking Ahead
Stakeholders should consider several outstanding questions and potential implications stemming from the proposed FY 2023 changes for existing assets and for future cell and gene therapies.
- Stability of MS-DRG 018: The proposed FY 2023 base rate for MS-DRG 018 is generally aligned with FY 2022 levels, though changes to the outlier threshold could result in overall decreases in payment. Total reimbursement will vary by hospital and case, with adequate reimbursement in some cases but with potential financial risk for hospitals on significantly costly cases. In the future, inclusion of additional immunotherapies that could be mapped to MS-DRG 018 may lead to fluctuations in the base rate. Several stakeholders have also submitted comments raising concerns about how the CMS would address an inpatient gene therapy that maps to MS-DRG 018 but has significantly different costs. With a robust pipeline of cell and gene therapies, the CMS may be forced to confront reimbursement challenges or alternative approaches in the near future.
- Combined NTAP Consideration: In cases where 2 products under consideration for NTAP are viewed as substantially similar (i.e., have the same targeted therapeutic outcome, same or similar mechanism of action, and map to the same MS-DRG), the CMS may consider those products as a single application for the purposes of NTAP. In this proposed rule, the CMS is collecting feedback on whether CarvyktiTM (ciltacabtagene autoleucel; NTAP requested) and Abecma® (idecabtagene vicleucel; existing NTAP) are substantially similar. If the CMS determines that they are, the decision could narrow the period of NTAP eligibility for CarvyktiTM and may impact the maximum NTAP for both products. As more CAR-Ts come to market, it may become increasingly difficult for manufacturers to demonstrate NTAP eligibility based on established criteria and providers may be less likely to benefit from max NTAP payments.
- Alternative Payment and Value-Based Arrangements: Starting on July 1, 2022, the Medicaid Value-Based Purchasing flexibilities to implement value-based arrangements in the commercial and Medicaid markets will go into effect. In the coming years, the CMS may consider whether innovative financing approaches should extend to the Medicare Fee-for-Service or Medicare Advantage space, potentially through a Center for Medicare & Medicaid Innovation demonstration.
- Potential for Shifts in Site of Care: As CAR-T treatment toxicity profiles increasingly make a move to outpatient sites of care more viable, providers may face different financial risk considerations. However, a shift in volume toward the outpatient setting may also result in increased scrutiny on Medicare payment in that setting, potentially resulting in changes to current reimbursement, which is average sale price plus 6 percent for separately payable drugs.
To stay up to date with the latest developments in the IPPS proposed rule, connect with us.
January 23, 11 AM ET
Learn More


