CAR-T Reimbursement Updates Proposed for FY 2024
Summary
Policy proposals for Medicare’s CAR-T inpatient reimbursement reflect additional cases and increase clinical trial payment.The Centers for Medicare & Medicaid Services (CMS) proposes to continue the use of its Medicare Severity Diagnosis-Related Group (MS-DRG) for chimeric antigen receptor T-cell (CAR-T) treatment stays. Differential reimbursement will be provided based on whether the product was provided as part of a clinical trial, with large increases proposed this year. The financial impact of changes in the fiscal year (FY) 2024 Inpatient Prospective Payment System (IPPS) proposed rule will vary by hospital, and reimbursement may continue to fall short of fully recognizing provider costs of treatment in some cases.
Background
Since the first Food & Drug Administration (FDA) approval of a CAR-T product in 2017, concerns have persisted over how the Medicare program would reimburse for these products, which are commonly administered in the inpatient setting and have a significant cost for providers (e.g., average sales prices exceeding $400,000 for several products). Hospital inpatient reimbursement is calculated on a case basis using an MS-DRG base payment rate that is adjusted for factors such as hospital geography, diagnosis, case severity, and discharge status. Additional reimbursement can be provided through new technology add-on payments (NTAPs) and outlier payments.
For FY 2023, inpatient stays with CAR-T treatment are currently assigned to MS-DRG 018, which has a base reimbursement rate of $247,939. Hospitals currently receive additional payments for three products with NTAP status (TecartusTM, Abecma®, and CarvyktiTM). The NTAP, however, is limited to a maximum of 65% of the product cost and varies based on the hospital’s case cost compared to MS-DRG payment. Outlier payments are available to hospitals to cover extremely costly cases in which the costs exceed the MS-DRG payment, the NTAP amount (if applicable), and the current fixed-loss threshold of $38,788. Even with these adjustments, Medicare reimbursement for CAR-T cases today sometimes fails to cover total hospital costs, with potential negative impacts on provider uptake and patient access.
Proposed FY 2024 Changes
For FY 2024, CMS proposes several policies that would impact provider reimbursement for CAR-T.
- Payment Changes for CAR-T Cases: As a result of an increase to the base operating and capital rates for all IPPS payments and an increase in the proposed relative weight for MS-DRG 018, the proposed base payment for CAR-T cases in FY 2024 would increase by 6.4% to $263,744.
- High-Cost Outlier Payments: CMS proposes to return to its historical method of establishing the fixed-loss threshold in a way that does not adjust for COVID-19 cases (as was done in FY 2023). The proposed fixed-loss threshold for FY2024 is $40,732, a 5% increase. For CAR-T cases, which are more likely than other inpatient stays to qualify for outlier payments, this will require hospitals to incur more losses before qualifying for an outlier payment, but the higher overall MS-DRG base rate should mitigate negative impacts.
- Adjustment for Clinical Trial Cases: CMS reimburses CAR-T clinical trial cases, which do not incur drug costs, at a lower rate than non-clinical trial cases. Using its standard approach to analyze 2022 Medicare Provider Analysis and Review (MedPAR) data, CMS found that clinical trial cases for CAR-T treatment incur 28% of the cost for non-clinical trial cases. Therefore, the agency proposes an adjustment factor of 0.28 to the relative weight of MS-DRG 018 for these cases (an increase over the current adjustment factor of 0.21). This would result in a base rate for clinical trial cases of $73,848, an increase of 42%. This increase reflects updated 2022 claims data, which show much higher average case costs for clinical trial cases than those reflected in the 2021 data. Additionally, CMS proposes changes to identifying CAR-T clinical trial cases to conduct rate setting for MS-DRG 018 by leveraging a combination of diagnosis codes and condition codes on claims.
- Product NTAP Decisions: In this proposed rule, CMS did not evaluate any new CAR-T products. CMS notes that NTAP payments for three CAR-T products will expire in FY2024: Abecma (idecabtagene vicleucel), Tecartus (brexucabtagene autoleucel), and Carvykti (ciltacabtagene autoleucel). Though Carvykti only qualified for NTAP payments starting in FY 2023, the window of eligibility was shorter as CMS deemed the product to be “substantially similar” to Abecma, meaning its newness period was tied instead to the FDA marketing authorization date for Abecma.
- NTAP Eligibility Changes: CMS proposes changes to two requirements for product NTAP eligibility, which could impact future NTAP submissions for CAR-T products:
- CMS proposes requiring applicants to submit their FDA market authorization requests by the time they apply for NTAP for FY 2024.
- CMS proposes moving the deadline for FDA authorization from July 1 to May 1 to allow more time to adequately assess the product for NTAP.
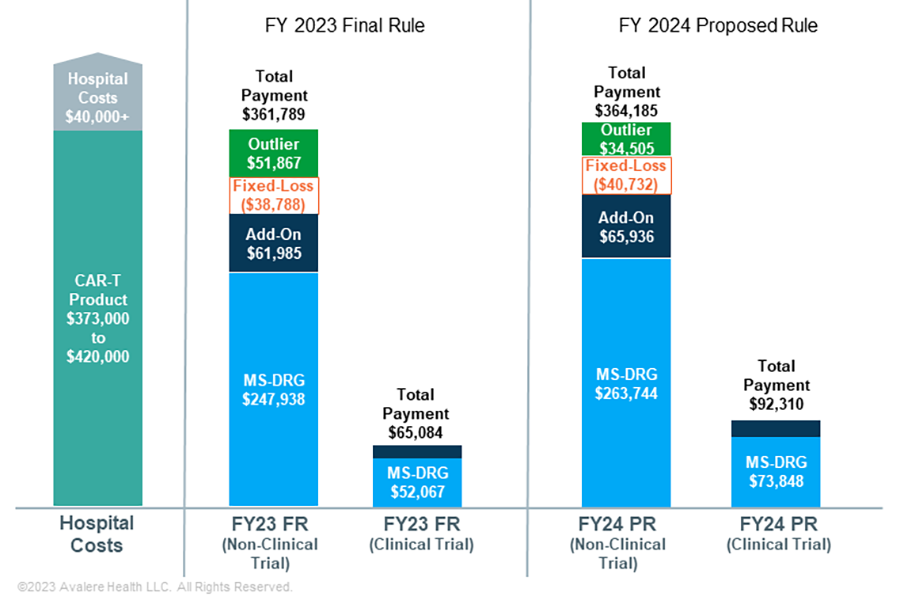
FR: Final Rule; PR: Proposed Rule. Figures not to scale. Assumptions: Hospital charges for CAR-T episodes are kept constant across all examples, consistent with the geometric mean charges included in the FY 2024 Proposed Rule after outliers removed file ($1,378,479); Hospital has an average operating and capital cost-to-charge ratio of 0.3; Hospital has an indirect medical education adjustment factor of 0.2 and disproportionate share hospital adjustment of 0.05; Hospital area wage index is 1.0
Key Considerations
Stakeholders should consider several outstanding issues and potential implications stemming from proposed FY 2024 changes for existing assets and for future cell and gene therapies.
- Stability of MS-DRG 018: While the proposed FY 2024 base rate for MS-DRG 018 represents a 6.4% increase over FY 2023, changes to the fixed-loss threshold may result in a more modest increase in cases that incur outlier payment. Total reimbursement will vary by hospital and case, with adequate reimbursement in some cases but potential financial risk for hospitals in significantly costly cases. The number of cases represented in the data to set the base rate nearly doubled this year (403 to 790), reflecting a larger number of on-market products and increases in utilization. In the future, the inclusion of additional immunotherapies, such as allogeneic CAR-Ts, that could be mapped to MS-DRG 018 may lead to fluctuations in the base rate. This may lead to the consideration of splitting the MS-DRG, depending on the number of cases and resource costs. With a robust pipeline of cell and gene therapies, CMS may have to confront reimbursement challenges or alternative approaches.
- NTAP Eligibility: Adjusting the newness criterion to move the FDA approval deadline up by two months, from July 1 to May 1, may impact the timeliness of reimbursement adequacy for providers and could have downstream implications for patient access. This means that a product launching on May 2 would not be eligible for NTAP until October of the following year, creating reimbursement uncertainty for providers over an extended period with potential negative effects on product uptake. Additionally, as more CAR-Ts come to market, it may become increasingly difficult for manufacturers to demonstrate NTAP eligibility based on established newness, cost, and clinical-improvement criteria, and hospitals may be less likely to benefit from NTAP payments.
- Alternative Payment and Value-Based Arrangements: Since initial market availability, value-based arrangements for CAR-T products have been considered given their high costs and therapeutic promise. However, regulatory and operational barriers have limited progress in establishing these arrangements across payer markets. Notably, the Department of Health and Human Services recently directed the Centers for Medicare and Medicaid Innovation (CMMI) to advance a voluntary model aimed at cell and gene therapies in Medicaid that would allow CMS to negotiate and administer outcomes-based agreements on behalf of states in certain therapeutic areas. Model details are expected in 2024 and 2025, with a model launch expected as soon as 2026. Additionally, CMMI is instructed to consider further research into alternative reimbursement approaches in Medicare fee-for-service (FFS) for cell and gene therapies, such as bundled payments or site-neutral payments. This would represent a significant shift in the Medicare FFS space, though no timeline for implementation has been provided.
- Potential for Shifts in Site of Care: Differences in Medicare reimbursement methodology in the inpatient versus outpatient settings generally result in more adequate reimbursement for CAR-T in the outpatient setting. Assuming that a CAR-T therapy can be safely administered in an outpatient setting, shifts toward the outpatient setting may increase scrutiny on Medicare’s outpatient drug payment methodology, which is currently the average sales price plus 6% for separately payable drugs.
Manufacturers of cell and gene therapies, along with other stakeholders including providers and plans, should carefully monitor reimbursement for these innovative products and engage stakeholders based on anticipated developments. For questions or to discuss potential Avalere support related to commercialization, NTAP proposal submissions, provider reimbursement, or policy developments, connect with us.
January 23, 11 AM ET
Learn More



