Expanding Patient Access to Digital Mental Health Services & Products
Summary
The COVID-19 pandemic is driving greater demand for digital mental health products and services. Stronger scientific evidence is necessary to determine value and increase payer reimbursement.Although 1 in 5 US adults live with mental health issues, fewer than half of those receive care in a given year. Some of the major barriers to care—such as provider shortages, affordability, and stigma— may be mitigated by the recent surge in telehealth and digital tools. The flexibility and continuity of care that telehealth offers can help expand access to mental health treatment and provide support to patients in the privacy of their own homes.
Mental Health: There’s an App for That
Given that the National Council for Behavioral Health estimates that 1 in 3 Americans fear judgment for seeking mental health services, the need for discreet options for mental healthcare is immense. App-based mental health options span the continuum of care and offer various technological features. It is not a 1-size-fits-all approach. The degree to which providers directly engage with patients also varies; apps may be consumer driven or provider driven (i.e., involve provider interaction).
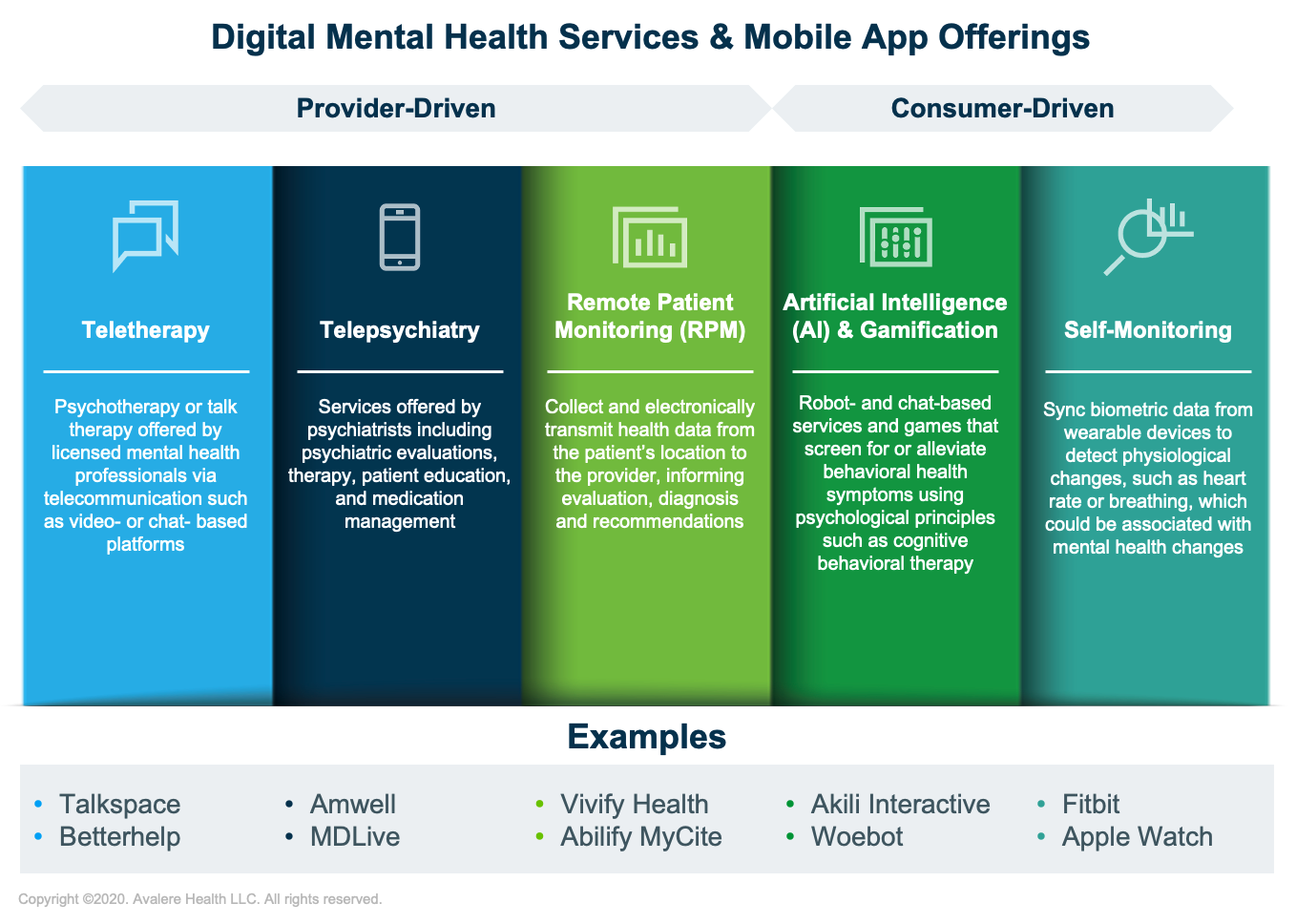
The costs for digital mental health applications vary across the spectrum of offerings. While many consumer-driven resources are accessible for free or for a single upfront cost, service-based sessions offered via provider-driven apps are often subscription based or charged per session (Table 1).
| Cost Structure | Teletherapy | Telepsychiatry | RPM | AI & Gamification | Self-Monitoring |
|---|---|---|---|---|---|
| Free or 1-time cost | X | X | |||
| Subscription based | X | X | X | ||
| Payment per session or service | X* | X* | X |
* Due to COVID-19, many payers have eliminated beneficiary cost-sharing for all mental health visits.
The actual out-of-pocket cost to the patient differs across digital services. Whereas most provider-driven services are reimbursed by insurance companies, most consumer driven applications are not covered. Similar to drugs, insurance coverage of digital services relies on FDA approval, which requires evidence demonstrating safety and effectiveness. While patient copays or coinsurance are often required for digital services covered by insurance, many payers have temporarily eliminated beneficiary cost-sharing for all mental health visits due to COVID-19.
Follow the Evidence to Enhance Reimbursement
Widespread acceptance and payer coverage of apps significantly depends on evidence generation. While the Food & Drug Administration (FDA) periodically updates guidance for mobile medical applications and has a voluntary program to pre-certify organizations developing medical software, not all provider- and consumer-driven apps require FDA approval prior to marketing. As a result, many apps are created with limited or no evidentiary support.
The intended use of an app determines whether FDA oversight is necessary and is defined by language used in labeling claims, advertising materials, or any statements made by the developers. For example, apps that purport to diagnose, cure, mitigate, treat, or prevent an illness qualify as medical devices, thus requiring FDA oversight. Other devices and general wellness products may be excluded from FDA oversight based on their intended use and whether they are considered low risk to patients. Of note, in response to COVID-19, the FDA has temporarily reduced regulatory oversight of apps that treat psychiatric disorders that typically fall within the FDA’s definition for medical devices and are deemed to pose a low risk to the public.
More than 10,000 behavioral health apps are available via Apple App Store and Google Play, but the clinical evidence to support most of them is limited. Mental health apps often include scientific language to support claims, even though most apps lack real-world data to quantify effectiveness.
Real-world evidence is needed to support credibility among patients, providers, and payers, ultimately to ensure that patients have meaningful access to care. Without strong evidence, Medicare and other payers are unlikely to issue coding to support coverage and payment for these types of digital services and apps. In June 2020, the FDA approved the first game-based digital tool. To obtain approval for EndeavorRx to treat children with ADHD, Akili Interactive submitted data from multiple studies, including a peer-reviewed randomized control trial. While not currently covered by plans, the company is working to increase payer coverage. Proactive engagement with payers in tandem with the FDA approval process is necessary to enhance payer coverage.
Future Outlook
The long-term impact of COVID-19 on mental health status is yet to be determined, but early research suggests the demand for behavioral health services will increase. A majority of payers have waived patient cost-sharing for tele-behavioral health services during the pandemic. The Centers for Medicare & Medicaid Services and some private payers have temporarily expanded telehealth reimbursement for a range of services, including psychiatry and psychotherapy, and some of these changes are expected to remain. However, coverage for digital health service apps outside of telehealth and remote patient monitoring is largely unchanged and will likely remain that way without the generation of necessary evidence.
Further, the FDA has temporarily relaxed requirements for apps used to treat psychiatric disorders due to the public health emergency. The FDA’s Digital Health Innovation Action Plan suggests it will continue to refine its regulatory policies to drive efficiency and build expertise in evaluating digital health products. Collectively, these tailwinds have primed the market for the expansion of digital health services, and the industry should look to produce evidence to address coverage gaps and expand patient access.
To receive Avalere updates, connect with us.
January 23, 11 AM ET
Learn More



