MA Plans Increase Use of Step Therapy for Part B Drugs
Summary
In 2023, 54% of Medicare Advantage enrollees were in plans that required step therapy for 10 commonly used rheumatoid arthritis drugs covered under Part B.Background
A variety of specialty drugs are used in the treatment of rheumatoid arthritis (RA) to control its progression and symptoms. These include disease modifying anti-rheumatic biologics, which play a pivotal role in improving the prognostication of the disease and patient quality of life. Currently, RA biologics may be covered either under Medicare Part B or Part D, depending on whether the drugs are administered within an outpatient care setting or self-administered by a patient. In Part B fee-for-service (FFS), all Food and Drug Administration-approved RA drugs are covered when reasonable and necessary, permitting physicians to select the treatment that they believe will be most effective at allowing a patient to meet their treatment needs and goals. In contrast, Medicare Advantage (MA) plans are increasingly using various tools at their disposal to control utilization and spending of RA drugs.
Since 2019, the Centers for Medicare & Medicaid Services (CMS) has allowed MA plans to require step therapy (ST) for Part B drugs for patients beginning a new course of treatment. MA prescription drug plans (MA-PDs) may require patients to step through a Part D drug therapy prior to allowing a Part B drug therapy, or vice-versa, and all MA plans (including MA-PDs) can require patients to step through one Part B drug before gaining access to another. CMS issued the guidance in expectation that allowing MA plans more flexibility in controlling Part B product utilization would enable them to negotiate more effectively with manufacturers. CMS issued final regulations, which became effective in 2020, formalizing MA plans’ ability to require ST for Part B drugs.
Despite increased plan flexibility regarding ST for Part B drugs, stakeholders have expressed growing concern over the potential for inappropriate denials of services as MA plans increasingly employ not just ST, but other UM requirements such as prior authorization (PA). Critiques about inappropriate UM practices are reflected in a 2022 Department of Health and Human Services Office of the Inspector General’s report, which found that MA plans sometimes denied or delayed beneficiaries’ access to services, even if the requests met Medicare FFS coverage requirements.
In response to concerns, in February 2024 CMS finalized policies that increase transparency surrounding MA plans’ PA requirements for non-drug items and services and shorten the timeframe in which a PA decision must be made. This rulemaking underscores an increased urgency across stakeholders and CMS’s willingness to promote transparency and oversight of the MA program. In the most recent rule, however, the agency stopped short of exploring specific guardrails related to ST and drug access.
Analysis of Utilization Management Strategies
Avalere evaluated MA plans’ annual medical policy and formulary restrictions data for 22 RA drugs covered under MA from 2018 to 2023.
Use of Step Therapy by MA Plans
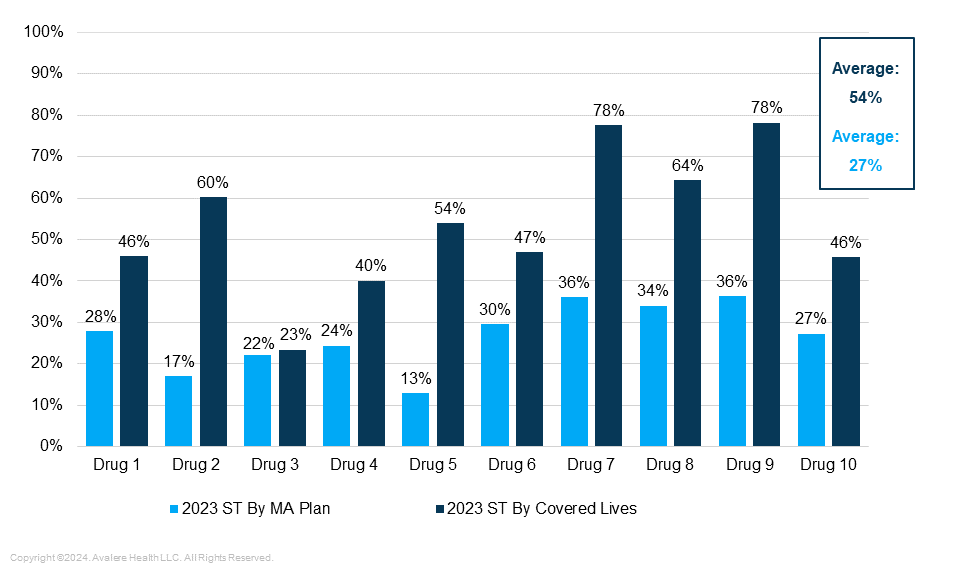
- Since 2019, when CMS gave MA plans the ability to use step therapy for Part B drugs, the proportion of enrollees in plans that apply ST to the analyzed set of RA drugs has increased steadily every year. By 2023, more than half of MA enrollees (54%) were in plans that applied ST to the analyzed set of 10 commonly used physician administered RA biologics under the medical benefit (Figure 1).
- For two of the products studied, 78% of enrollees were in MA plans that applied step edits to these products in 2023.
- Across MA plans that required ST for RA drugs under the medical benefit, an average of 27% of enrollees were in plans requiring two or more steps—but that share could be as high as 44% of enrollees for specific drugs.
Figure 1: Percent of Plans and Enrolled Lives With Step Therapy for a Given RA Drug Under Medical Benefit, 2023
Cross-Benefit Management
- MA-PD plans that manage drug access through a cross-benefit approach mostly required stepping over a Part D drug before gaining access to prescribed Part B drugs, rather than the inverse. MA-PD plans imposed ST restrictions that required enrollees to step through a pharmacy benefit drug before a medical benefit drug 51% of the time in 2020; this proportion fell to 40% in 2023.
- Conversely, the proportion of enrollees in MA-PD plans that required stepping over Part B RA drugs to get access to Part D products remained minimal and largely unchanged, rising from 4% in 2018 to 6% in 2023.
Utilization Management for Biosimilars
- MA plans increased ST protocols referencing biosimilars since the commercialization of key products starting in 2020. By 2023, about a quarter of MA plans on average applied ST protocols to Part B RA biosimilars.
Key Considerations
The share of beneficiaries in MA relative to FFS continues to increase, calling attention to access to Medicare-covered items and services by MA plans. These findings offer insight into how plans currently apply UM tools on some medical benefit drugs and how the application of UM has changed since the effectuation of CMS’s guidance.
The increased use and changing application of ST in MA have implications for beneficiary medication access. Changes in utilization management in MA have come under increased scrutiny in recent years, with some stakeholders calling for increased protections regarding UM criteria to ensure that beneficiaries in MA plans have the same access to clinically appropriate care as they would in FFS and do not face disruptions, delays, or adverse events. In particular, the growing trend to manage RA drugs between Part B and Part D has called into question whether CMS has the appropriate tools to review cross-benefit UM and ensure it is in line with FFS coverage requirements and it sufficiently accounts for individual patient needs.
Looking ahead, the Inflation Reduction Act (IRA) includes several policies that could impact MA plan liability, including Part D benefit redesign and Medicare negotiation, which may lead MA plans to find new ways to manage drug spending and control costs. As the IRA continues to be implemented, stakeholders and policymakers should monitor closely the balance between MA plan activities to manage enrollee care with the monitoring and oversight that CMS is willing to do with respect to the clinical appropriateness of cross-benefit step edits and UM.
Methodology
Avalere partnered with Clarivate to obtain formulary and policy restrictions data for coverage analyses. Drugs were categorized as a medical benefit (i.e., physician administered drugs) or pharmacy benefit (i.e., self-administered drugs) based on CMS spending data and drug administration.
Dive Deeper
To learn more about the impact and prevalence of UM in Medicare, as well as the interaction of IRA implementation with MA, connect with an Avalere expert today.
Funding for this research was provided by PhRMA; Avalere retained full editorial control.
Webinar Replay | Election 2024: What's at Stake for Healthcare?
In this webinar, Avalere experts and guests discuss the 2024 elections, exploring the candidates’ health policy approaches and implications for stakeholders.




