Navigating the Road to RSV Prevention
Summary
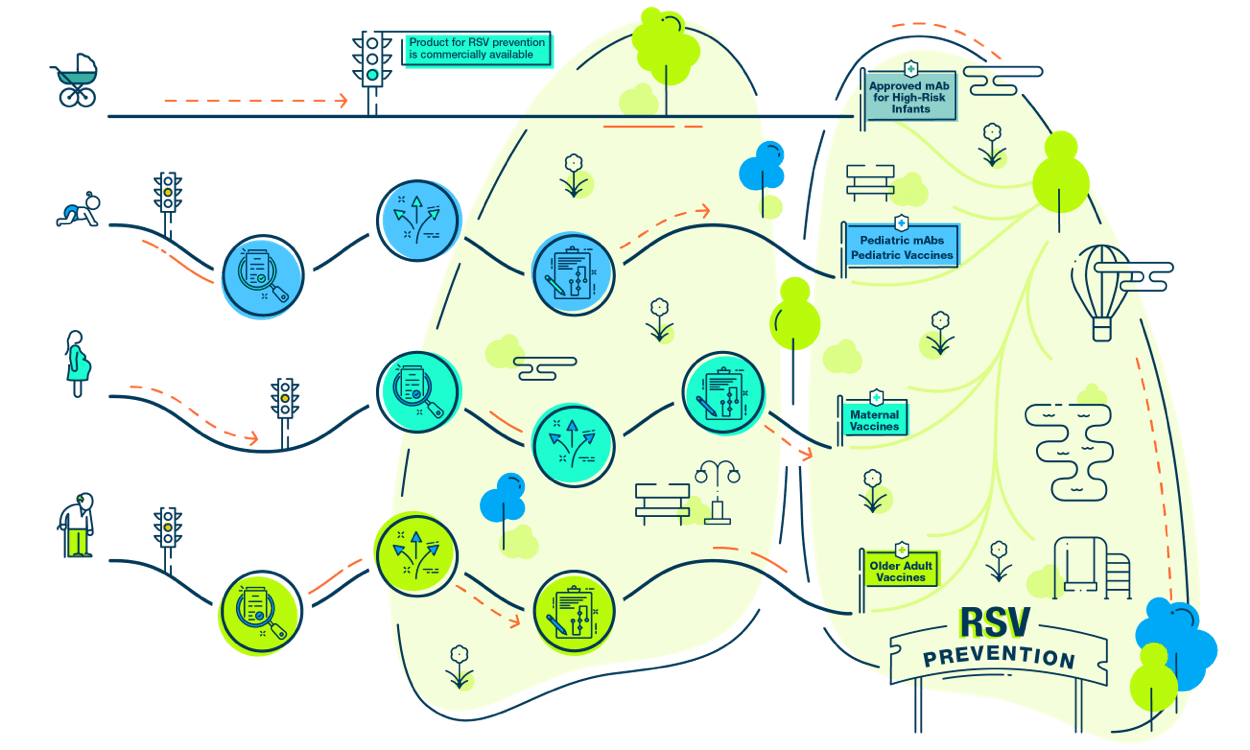
Numerous novel respiratory syncytial virus (RSV) preventive products, including vaccines and monoclonal antibodies (mAbs), are in late-stage clinical development. Consequently, the RSV prevention landscape is expected to transform in the coming years. Adequate preparation to incorporate the use of these new tools should consider the regulatory and recommendation pathways and their implications for coverage and reimbursement.Since the discovery of RSV in the 1960s, lower respiratory illness disease burden has consistently increased globally. On average, RSV leads to approximately 2.1 million pediatric outpatient visits and more than 170 thousand hospitalizations among adults 65 years of age and older. Currently, no vaccine is available to prevent infection.
Fortunately, through continued research, a non-vaccine preventive product, palivizumab, was approved in 1998 and is part of a therapeutic group of products with the potential to prevent RSV infections. More recently, a significant number of vaccine-like antibody products as well as vaccines are under development and in testing phases. These products have the potential to come to market in the coming years. All of these products will be novel and will require adaptations to the immunization system. They will have unique coverage and access considerations as well as uptake barriers.
- RSV monoclonal antibodies will be the first preventive monoclonal antibodies intended for routine use in a broader population of infants.
- RSV maternal vaccines will be the first vaccine intended to be given to pregnant individuals primarily for the protection of the neonate or infant.
- RSV older adult vaccines will add to the growing pipeline of adult life-course vaccines and have the potential to be combined with the influenza or COVID-19 vaccine as novel combination vaccines.
- RSV pediatric vaccines will be the first pediatric vaccine to be launched after another preventive product is being used routinely.
Avalere can help organizations and companies address the roadblocks they may encounter on the pathway to developing next-generation RSV prevention products.
Pathways to RSV Prevention
Click on the orange circles with the plus sign to read more about each step.