USP DC 2025 Updates and Strategic Engagement Opportunities
Summary
USP’s updated Drug Classification for plans outside of the Medicare Part D market reflects revisions and newly approved products through October 2024.The final US Pharmacopeia Convention (USP) Drug Classification (DC) 2025, released on January 15, 2025, refines drug classifications and adds FDA-approved drugs through October 2024 for health plans’ formulary planning.
The USP is a nonprofit organization that disseminates public compendial quality standards for medicines and maintains two drug classification systems: the Medicare Model Guidelines (MMG) for Part D plans and the DC for non-Part D plans. These classification systems group eligible drugs into categories and classes that health plans can use in their formulary development process. While there are limited formal requirements regarding how health plans interact with the USP’s classification systems, the USP’s decisions on how drugs are grouped have downstream impacts on plan formularies, coverage requirements, and patient access.
The USP Releases Two Classification Sets to Guide Formulary Development
The Medicare Prescription Drug Improvement and Modernization Act of 2003 requires the USP, in collaboration with stakeholders, to create a list of categories and classes for Medicare Part D drug formulary development. CMS contracted with USP to draft the MMG to serve this purpose and update drugs and classifications on a three-year schedule.
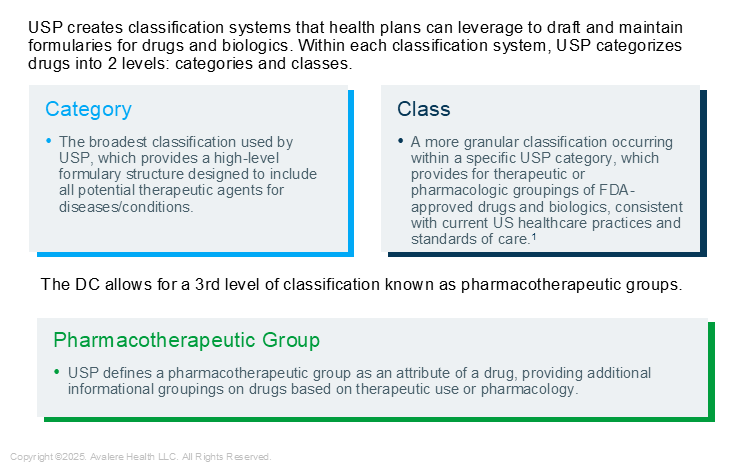
At the request of health plans with other books of business (i.e., non-Medicare Part D plans), the USP launched the first version of the DC in 2019. The DC includes medications beyond those specifically covered under the Medicare Part D benefit—such as drugs used to treat symptomatic relief of cough and cold and to promote fertility—and is more comprehensive than the MMG. Unlike the three-year update cycle of the MMG, the DC is updated annually, offering stakeholders more opportunities to engage the USP. In addition, the USP DC includes a third level of granularity to drug classification, Pharmacotherapeutic Groups (PGs), which offers additional opportunity for differentiation.
Figure 1. USP Classification Systems
Notable Changes Between the USP DC 2024 and 2025
The following changes were made from DC 2024 to DC 2025:
New Class:
- 1 new class: “Erythropoiesis Promoters” in the “Blood Products and Modifiers” category.
New Pharmacotherapeutic Groups (PGs):
- 65 new PGs were added across various categories, with notable increases in the Antineoplastics category, including 34 PGs in the Molecular Target Inhibitor class and 24 PGs in the Monoclonal Antibody/Antibody-Drug Conjugate class.
- 7 new PGs were added under the Blood Products and Modifiers category, including new groups in Erythropoiesis Promoters and Blood Component Deficiency/Replacement.
Changed Classes and PGs:
- 1 class renamed: “Treatment-Resistant” in the Antipsychotics category is now called “Antipsychotics, Other.”
- 1 PG renamed: In the Respiratory Tract/Pulmonary Agents category, the “Conductance Regulator Potentiators” PG is now “Transmembrane Conductance Regulator Modulators.”
Removed PGs:
- 2 PGs removed from the Blood Products and Modifiers, Other class: “Erythropoietins” and “Blood Products and Modifiers, Miscellaneous.”
- 1 PG removed from the Immunosuppressants class: “Interleukin-2 Inhibitors.”
Figure 2. The USP MMG and DC

* Based on communication with the USP, work on MMG v10.0 will likely begin in 2026, with final timelines contingent on agreements with CMS. MMG v10.0 is anticipated to be released in the fall of 2026, and USP expects v10.0 will be effective from PY 2027-2029. However, given that formularies must be submitted to CMS by the first Monday in June for the subsequent plan year, Avalere expects MMG v9.0 to be effective through PY 2027 and MMG v10.0 to be effective from PY 2028 to 2030.
Based on communications with USP, Avalere anticipates the next open engagement period to be on schedule for USP DC 2026 (October 2025). Beyond that, we anticipate USP DC 2027 and USP MMG v10.0 to be released in Fall 2026. While the USP does not offer a formal appeal process, stakeholders can take proactive steps to ensure their input is considered during upcoming updates.
Stay Informed
- Understand Current Positioning: Reviewing the current classification of relevant drugs in USP DC 2025 and MMG v9.0 helps identify questions and develop a strategy for engagement.
- Monitor Release Schedules: The USP updates the DC annually, typically releasing a draft in October with an accompanying public comment period. Awareness of these timelines ensures timely participation.
Be Prepared
- Prepare Documentation: Collect comprehensive evidence and documentation to support proposed classification changes.
- Strategic Planning: Develop a well-founded engagement strategy that identifies key issues and incorporates evidence-based arguments.
Engagement with USP
- Request Meetings: During open engagement periods, stakeholders can request one-on-one meetings with the USP to discuss classification concerns or seek clarification on criteria.
- Participate in Public Comments: Submitting well-crafted feedback during public comment periods ensures alignment with USP requirements and effectively communicates key positions.
Collaborate with Avalere for Strategic USP Support
Avalere’s experts in prescription drugs and formulary systems support clients in analyzing USP classification updates, identifying strategic opportunities, and influencing future editions of the USP Drug Classification. We partner with clients to ensure classifications reflect current medical practices and enhance patient access to therapies.
To learn more about how Avalere can support your engagement with the USP, connect with us.






