Wastage Update: New JZ Claims Modifier Applies to NOC Billing
Summary
Starting July 1, providers must report the JZ modifier on all claims for single-use Part B drugs when applicable, including for products billed with an NOC code.In previous Insights on the operationalization of the discarded drug refund provision, Avalere identified opportunities for stakeholders to engage the Centers for Medicare & Medicaid Services (CMS) and assessed strategic planning implications for affected products. In this installment, Avalere experts provide a closer look at recent modifier implementation developments.
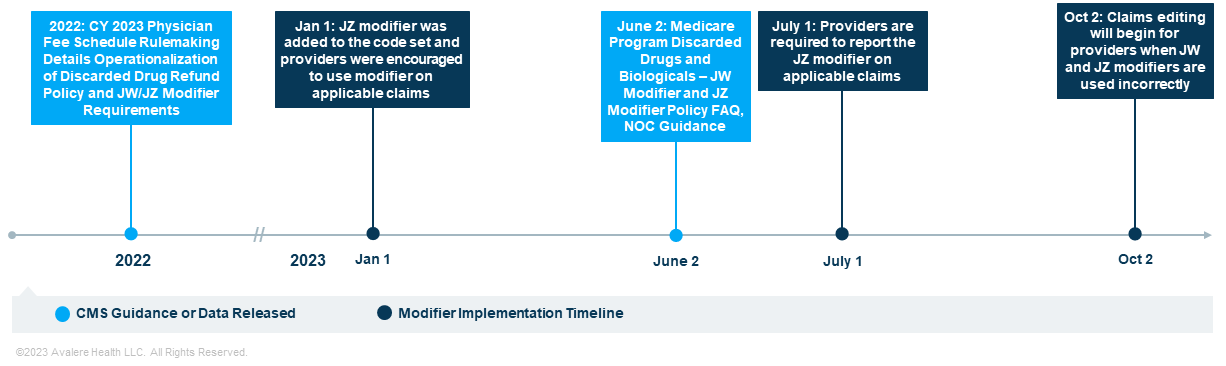
In the Calendar Year 2023 Medicare Physician Fee Schedule final rule, CMS operationalized a provision included in the 2021 Infrastructure, Investment, and Jobs Act that required manufacturers to provide quarterly refunds to CMS for any amount of discarded single-dose vial or single-use drug over 10% of the total allowed amount beginning January 1, 2023. Through this subsequent rulemaking, CMS also codified the requirement to use the JW and JZ modifiers for single-dose container drugs separately payable under Part B to track and calculate discarded drug refunds effective January 1, 2023. While the JW modifier differentiates the discarded product from the quantity administered to patients, Medicare continues to reimburse providers for the entire single-use vial.
| Modifier | Short Descriptor | Long Descriptor |
|---|---|---|
| JW | Discarded drug not administered | Drug amount discarded/not administered to any patient |
| JZ | Zero drug wasted | Zero drug amount discarded/not administered to any patient |
New JZ Modifier Required July 1, 2023
CMS has implemented the JW and JZ modifiers in phases (Figure 1). The JZ modifier was available for use beginning January 1, 2023. Effective July 1, however, Part B providers using single-dose vials must report the JZ modifier on all claims when there is no discarded amount from single-dose containers or single-use packages. For the amount administered, the claim line should include the following:
- The billing and payment code, such as a Healthcare Common Procedure Coding System code, describing the given drug
- The JZ modifier showing there were no discarded amounts
- The number of units administered
Historically, not otherwise classified (NOC) codes were believed to be excluded from the JW/JZ billing requirement. However, a recent CMS JW-JZ frequently asked questions document clarified that while NOC codes do not specifically identify a drug, for consistency with the policy, the JW and JZ modifiers are required to be reported for drugs from single-use containers billed with a NOC code (e.g., J3490, J3590, C9399). Since CMS does not use fractional billing units, it is assumed that the billing provider or supplier would report administering the full billing unit and the JZ modifier.
Have You Evaluated the Steps You Should Take?
Avalere’s robust billing and coding expertise and access to up-to-date discarded drug data can help manufacturers adapt to this new policy by understanding wastage for on-market products and providing insight for new launches. To learn how Avalere’s Part B reimbursement experts can help you assess your field training, effective provider-facing collateral, mitigation strategies, and impacts for stakeholders, connect with us.







