CMS Takes Steps to Expand Access to COVID-19 Antibody Treatment
Summary
The Centers for Medicare & Medicaid Services (CMS) released coding and payment instructions associated with the first physician-administered outpatient COVID-19 therapeutic. The release specifies that during the public health emergency (PHE), Medicare will cover and pay for these infusions in the same way it does for COVID-19 vaccines.Background
On November 10, following the announcement that the first physician-administered COVID-19 therapeutic had received an emergency use authorization (EUA) from the Food & Drug Administration for outpatient use, the CMS took actions to clarify the coverage, coding, and reimbursement for the drug in Medicare. The product is intended for the treatment of mild-to-moderate COVID-19 in adults and pediatric patients who are at risk of severe complications and hospitalization. The announcement follows the pandemic’s Fourth Interim Final Rule with Comment (IFC-4), which had included important payment provisions to outpatient hospitals for COVID-19 therapeutics.
The IFC-4 clarified that therapeutics specific to the treatment of COVID-19 will be paid separately regardless of the other services on the claim. This differs from the typical methodology where drugs can be bundled if provided on the same day as services paid via comprehensive ambulatory payment classifications.
Billing and Coding Details
The CMS’s announcement is aimed to facilitate quick access to the COVID-19 treatment in a wide range of settings. The CMS created two new Healthcare Common Procedure Coding System (HCPCS) codes, a Q code for the drug and an M code for the administration. Notably, the M code for the administration is unique to the infusion of the newly approved product and includes additional monitoring; however, the payment rate is set at the hospital outpatient department (HOPDs) rate for a 1-hour complex infusion. The release of the new HCPCS codes allows hospitals to accurately identify the product and payers to monitor its use. Additionally, the release may assure hospitals of coverage and payment for the product, which may help to increase patient access.
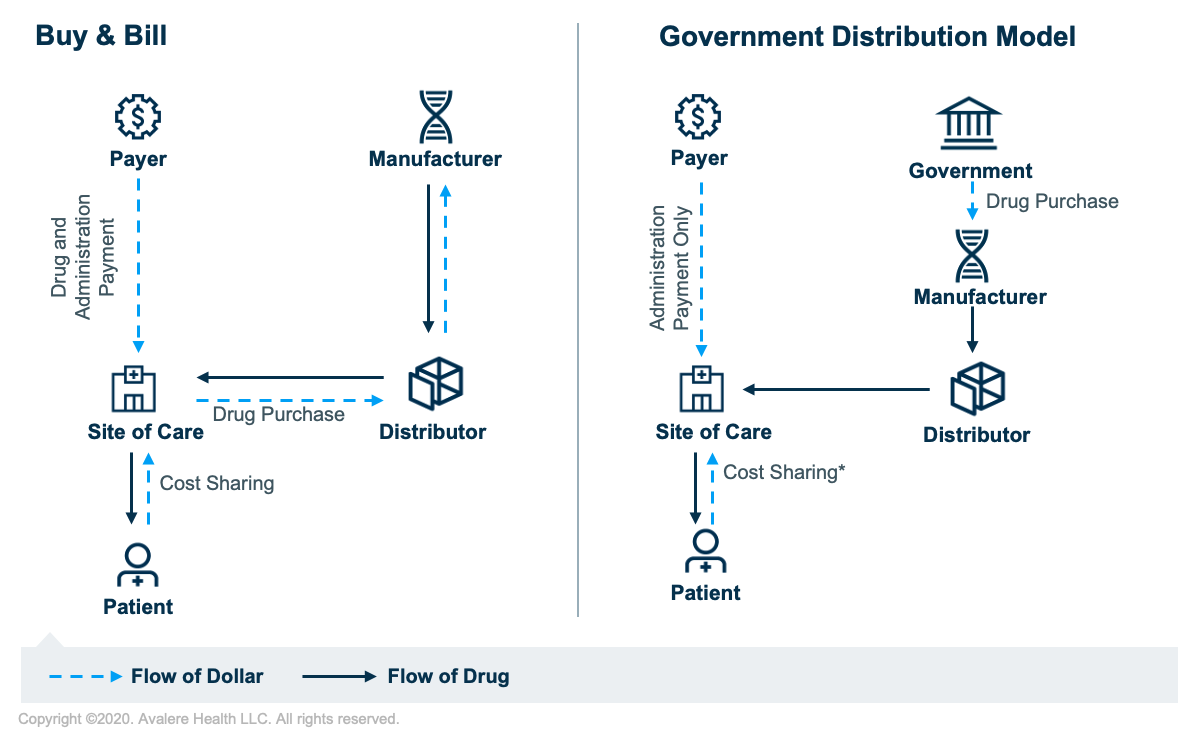
Drug Pricing and Purchasing Dynamics
Initially, providers will not be reimbursed for the drug itself, even though the CMS specified the Q code to be used for this new COVID-19 therapy. Instead, the government is purchasing an initial quantity of the product directly from the manufacturer and supplying it to providers for administration at no cost. This model mirrors the way the federal government negotiates for pediatric vaccines in public programs. Government purchasing and distribution for a product that is available under an EUA may allow for more expedited and equitable allocation of the therapies based on incidence or severity of COVID-19 outbreaks, but it circumvents the typical buy and bill methodology.
For the government purchasing where the hospital receives the drug at no charge, HOPD providers would report the HCPCS Q code for the COVID-19 therapeutic on the claim form with an associated “token” charge of $0.01 or $1.01 to signal no payment to Medicare. After this initial phase, hospitals will purchase the product and Medicare will pay 95% of the average wholesale price, which is how vaccines are currently paid.
*Zero dollar cost-sharing for Medicare beneficiaries for provider administration services
Patient-Cost Considerations
While there is no clear legislation around patient cost-sharing for COVID-19 therapeutics, the CMS announced within the coding and payment instructions that there will be be zero patient-cost sharing for COVID-19 therapeutics infusions in the HOPD. This somewhat contradicts the IFC-4, which specified beneficiaries receiving COVID-19 therapeutics, if purchased by the hospital, would be responsible for the 20% cost-sharing.
Additionally, it is unclear if the CMS intends zero cost-sharing on the drug itself or on both the drug and the administration, but the CMS noted they will address payment refinements in future rule making.
Implications of the Government Purchase and Distribution Model
The Biomedical Advanced Research and Development Authority announced the initial purchase of 300,000 doses of the product, with an option to purchase an additional 650,000 doses to allocate to state and territorial health departments, which in turn will determine which healthcare facilities receive shipments of the drug.
This is not the first time that the federal government has stepped in to facilitate product distribution during a PHE. However, this is the first COVID-19 outpatient intravenous infusion drug. Thus, unique distribution and supply considerations and different coverage and reimbursement factors were likely contemplated by the CMS before finalizing IFC-4. In the interim, this distribution model could potentially allow for quicker access and equitable allocation to address treatment needs for COVID-19. Nevertheless, further planning and guidance is necessary and critical to address ongoing supply, equitable access, and appropriate coverage and reimbursement for COVID-19 therapeutics and antibody treatment in the outpatient setting. While the new distribution and reimbursement model may set a new precedent, it is unclear how the government would handle other similarly high-priority products in the future. Finally, this temporary model could in theory also act as a yardstick to assess the feasibility of direct government involvement in Part B drug price negotiation, procurement, and reimbursement.
To learn more about Avalere’s COVID-19 work, connect with us.
January 23, 11 AM ET
Learn More



