New ESRD Analytics Featured in Kidney 360
Summary
On August 25, “Calcimimetic Use in Dialysis-Dependent Medicare Fee-for-Service Beneficiaries and Implications for Bundled Payment” was published in Kidney 360. The article featured research and analytics conducted by Avalere experts.The objective of this descriptive analysis was to understand the variability in calcimimetic use across key patient characteristics and its potential impact on policy options for incorporating the calcimimetics’ costs and payments permanently into the End-Stage Renal Disease (ESRD) bundle.
Dialysis-dependent patients with secondary hyperparathyroidism may require calcimimetics to help reduce elevated parathyroid hormone levels to treatment goals. Since 2018, Medicare has paid for the calcimimetic therapies via the Transitional Drug Add-on Payment Adjustment (TDAPA) designation under the ESRD Prospective Payment System. The calcimimetics have been the first products eligible for the TDAPA payment adjustment, and the Center for Medicare & Medicaid Services has proposed to conclude this payment designation and incorporate the calcimimetics costs into the bundle beginning in 2021. Concerns exist among stakeholders regarding disparities in calcimimetic usage and how the drug class will be accounted for in the ESRD bundled rate after the conclusion of TDAPA. Careful consideration will be required to ensure vulnerable patients requiring treatment for SHPT do not face barriers to appropriate care.
Key Findings from This Analysis
- The proportion of dialysis patients utilizing calcimimetics was roughly 80% higher in African-American versus non-African-American dialysis patients, 30% higher in dual-eligible versus non-dual-eligible dialysis patients, and 3 times higher in patients who had been on dialysis longer than 3 years versus those who had been on dialysis for less than 3 years
- Calcimimetic use was similar across census regions; however, substantial variation in calcimimetic utilization was observed at the facility level within specific analyzed metropolitan statistical areas
- Medicare spending for calcimimetic therapies as a proportion of total Medicare dialysis spending was greater than 10% in approximately 20% of dialysis facilities.
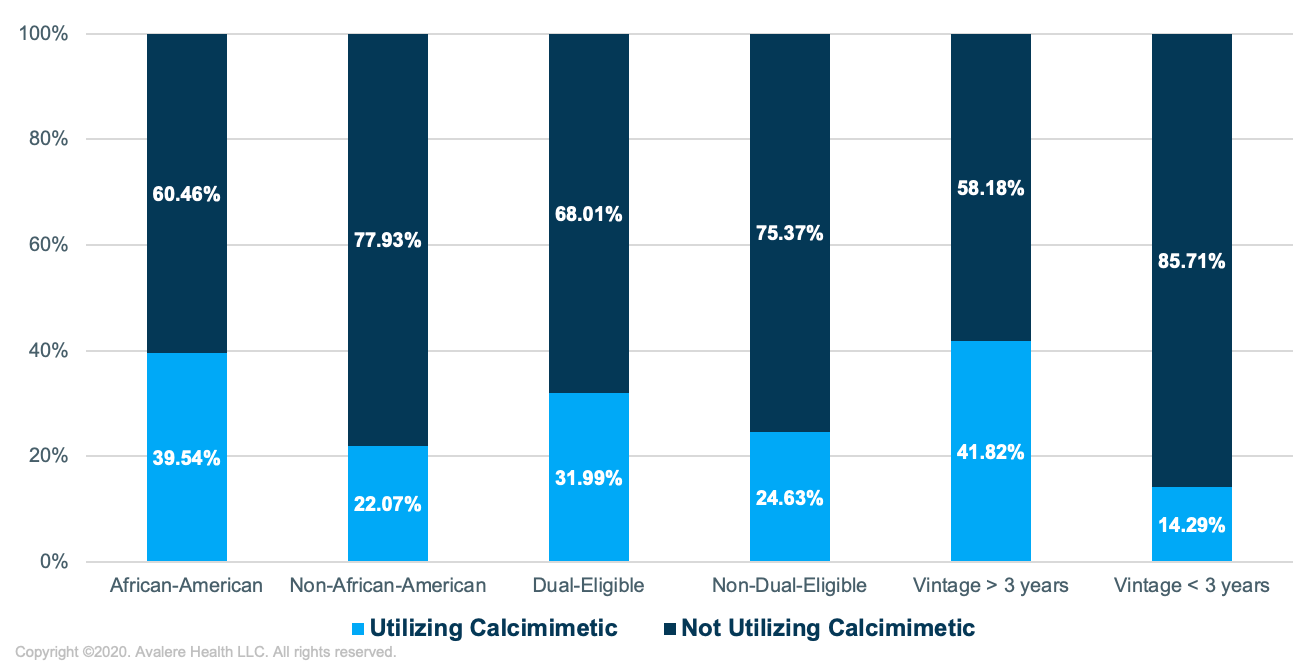
Note: Calcimimetic use is defined as having at least 90 days of calcimimetic utilization without a gap of no more than 60 days between any consecutive administrations.
Read the full report.
To receive Avalere updates, connect with us.
Funding for this research was provided by Amgen.







