Prescription Digital Therapeutics Bring New Treatments to Healthcare
Summary
Prescription Digital Therapeutics (PDTs) are a growing and unique treatment modality that can provide expanded options for treatment to patients. As the landscape for these treatments develop across multiple therapeutic areas, challenges related to coverage, reimbursement, and access will need to be solved to advance broader adoption and utilization across key stakeholders.Digital Health—Innovating Healthcare
Health systems are rapidly evolving to address issues that have been exposed and exacerbated by the COVID-19 pandemic. Mental health and healthcare disparities have intensified throughout the pandemic and are only growing as stakeholders grapple with adapting to these challenges. Healthcare technology has emerged as an important element to address these disparities and bring new methods of safe and effective treatment to patients. High-quality software tools and modalities can address the prevention, management, and treatment of chronic diseases and mental health disorders through non-invasive technology solutions. Through virtual 24/7 access and treatment availability independent of provider availability or geography, digital therapeutics present a unique opportunity to address health inequities and gaps in care.
In many cases, digital technologies have expanded the reach of clinicians’ ability to address disease by making the treatment process more convenient for patients. Options such as wearable devices, telehealth checkups, and remote monitoring have reduced many barriers to care, including travel and the need to take time off work, as well as the perceived stigma associated with seeking treatment for mental health disorders. However, many digital products rely significantly on the patient to use properly, and for certain conditions this level of self-management can be unrealistic. For instance, care transitions are critical moments for patients receiving treatment for substance use. Reduced touch points between intensive inpatient care to outpatient care settings can lead to patient drop out. In these cases, patients continue to require dedicated physician oversight. A variety of digital health modalities comprise the landscape of existing healthcare technology and are visualized in Figure 1.
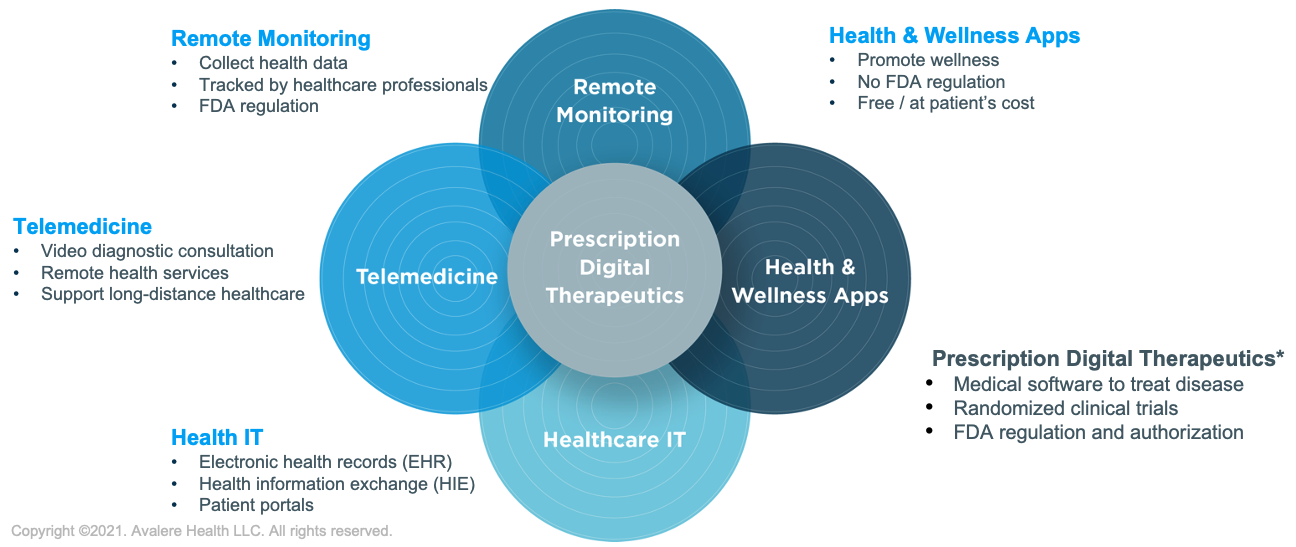
Reference: Digital Health Market: Market Segmentation, Global, 2017; recreated from Frost & Sullivan report: “US Digital Therapeutics Market, Forecast to 2023.”
PDTs–Distinguishing Features
PDTs are distinguished from other digital health technologies.
- PDTs are US Food & Drug Administration (FDA) authorized through the 510(k) premarket notification or de novo classification processes, having demonstrated clinical safety and efficacy.
- They are developed using regulatory standard good manufacturing practice.
- They require a prescription from a healthcare professional.
- They allow providers to monitor patients throughout the care continuum.
As the PDT landscape grows, however, coverage and reimbursement of PDTs remains a question for industry stakeholders. Avalere has teamed with Pear Therapeutics, an industry leader in PDTs, to help develop and publish the Pear Prescription Digital Therapeutics Digest, outlining current awareness, issues, and opportunities with PDTs, and how PDTs can benefit stakeholders across the healthcare system, especially patients.
While the PDT industry has experienced significant growth over the past several years, challenges still limit broader adoption. Such challenges include:
- Communicating perceived value
- Establishing clear coverage and reimbursement pathways
- Enhancing provider and patient-level of awareness and adoption
Survey Insights Illuminate Stakeholder Misconceptions and Opportunities
The Digest will incorporate findings from a survey of major US payers and employers on their perspective of the PDT market landscape. Variation in stakeholder responses regarding coverage and reimbursement pathways for PDTs confirms fundamental misconceptions on PDTs among key players in this space.
The survey results will highlight the following key findings:
- FDA authorization and evidence generation for PDT efficacy and value are an area of great interest for stakeholders
- Coverage and reimbursement pathways are unclear and may inhibit full use of these therapeutic options
- Opportunities are available to explore value-based contracting as a potential coverage mechanism for PDTs
- PDTs have the potential to add great value to patients as a complementary therapy for the treatment of complex diseases through improved patient adherence, higher quality care, and improved outcomes
PDTs are becoming a noteworthy part of the healthcare landscape, and stakeholder awareness, knowledge, and coverage of PDTs will be important to consider as more FDA-authorized products enter the market. The Digest and accompanying presentation at the Academy of Managed Care Pharmacy (AMCP) partner session “Pioneering Prescription Digital Therapeutics to Transform the US Healthcare System: PDT Digest Launch” featuring Avalere, will be launched at the AMCP Nexus on October 19.
To learn more about how PDTs are improving healthcare, connect with us.
January 23, 11 AM ET
Learn More



