The Rare Disease Market: Policy Changes and Coverage Trends
Summary
Changes to rare disease policies and payers’ approaches to coverage could have significant impacts on product development, patient access, and reimbursement.While the proportion of patients impacted by individual rare diseases is relatively small, the rare disease therapeutics market is growing, bolstered by transformative changes to research and development as well as modernized regulatory pathways. Payers and policymakers are considering changes to address the needs of this growing market and the challenges it may present.
With the growing number of drugs approved to treat rare diseases, payers are exploring various methods to address critical issues related to pricing, value, novel payment, and reimbursement mechanisms. At the same time, policymakers are discussing amendments to the Orphan Drug Act (ODA) that would create a new threshold for ultra-rare diseases and considering revising the use of the Food & Drug Administration’s (FDA) Accelerated Approval Pathway.
Background
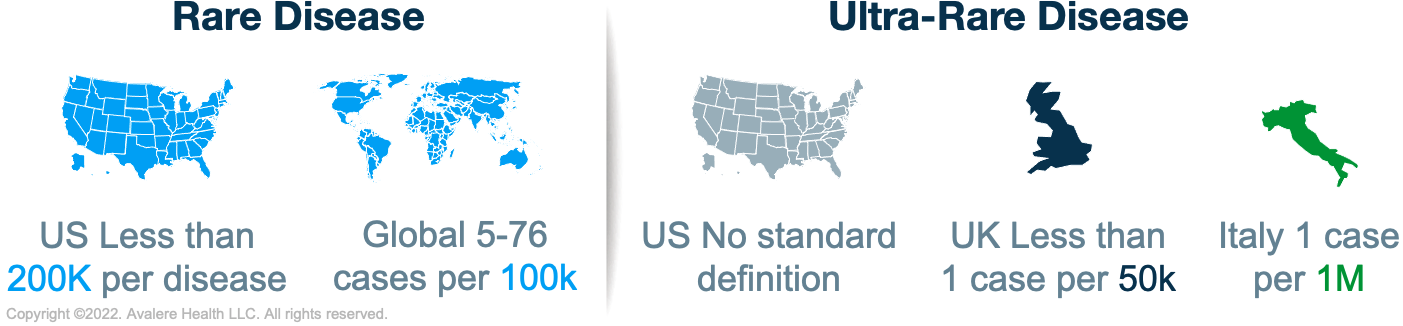
The ODA was enacted in 1983 to facilitate the development of pharmaceutical agents developed to treat rare and ultra-rare conditions. Prior to the ODA, only 38 orphan drugs had been approved in the US, compared to over 700 today. The enactment of the ODA set a global precedent for orphan drug development. Japan adopted the Orphan Product Development Support Program in 1999, which was followed by the EU Orphan Regulation in 2000. While the details of these policies vary, they share a common purpose: to stimulate the development of therapies for rare diseases by providing financial incentives, such as market exclusivity, to manufacturers. While the global regulations supporting rare-disease development have similar goals, their definitions for rare disease vary. The ODA was amended in 1984 to establish a definition for rare disease that remains today. The threshold for a rare disease in the US is defined as fewer than 200,000 people affected with a disease. Globally, prevalence thresholds for rare diseases vary from 5 to 76 cases per 100,000 people.
Pricing, Value, and Evidence
Payers are considering the role of value assessment and evidence generation for rare disease drugs in recent years due to increased spending on these products. A recent Institute for Clinical and Economic Review (ICER) white paper includes recommendations to address affordability of orphan drugs, including proposals to expand outcomes-based contracts (OBCs), value-based pricing, value-based contracts (VBCs), and other innovative contracting structures. Although payers are unlikely to fully adopt ICER’s recommendations and some use internal health technology assessment tools, ICER can influence payers’ final coverage decisions. However, as payers increasingly focus on evidence generation, they are becoming more interested in reforming the orphan drug reimbursement environment and contracting with manufacturers based on the value and outcomes that orphan drugs deliver. However, generating evidence can be especially challenging for orphan drug manufacturers because of small, geographically dispersed patient populations, significant disease heterogeneity, and, in many cases, rare disease progression mechanisms that are not fully understood. To compete in this new environment, orphan drug manufacturers will need to develop an evidence strategy early in the development process and build a value proposition that will resonate with payers.
Novel Financing Mechanisms
Rare disease drugs often have higher prices compared to non-orphan drug counterparts, and payer spending on these treatments can vary significantly from year to year, given the small patient population affected. Payers in all markets are seeking approaches that could ensure more predictable spending on orphan drugs. Some states have considered approaches to financing orphan drugs through VBCs, OBCs, or a rare-disease medication reinsurance program. Other innovative financing mechanisms for rare disease programs include public-private partnerships, voucher programs, and targeted “moonshot” grants, among others. Novel financing mechanisms that spread the risk across payers will play an important role in the broader coverage and availability of orphan drugs, particularly as more high-cost specialty drugs are introduced to the market. Orphan drug manufacturers will need to collaborate with payers when seeking innovative methods for contracting that will decrease the payer cost burden and optimize access to their products.
Accelerated Approval Pathway Updates
Rare disease research faces a particular challenge in generating statistically significant data from clinical trials due to small trial populations. One solution for sponsors developing therapies for rare diseases is to leverage the Accelerated Approval Pathway, which allows sponsors to use surrogate endpoints to show product effectiveness in order to provide earlier access to drugs and biologics that treat a serious or life-threatening disease. Sponsors then must confirm product effectiveness with further studies after the product is marketed. Recent congressional interest in the Accelerated Approval Pathway has led to advanced discussions about potential reforms, including increasing the FDA’s authority to withdraw approvals if confirmatory studies do not show effectiveness or endpoints are not met. These reforms could alter evidence generation expectations with the FDA and potentially make it more difficult for orphan drug sponsors to reach patients.
Differentiated Approach for Ultra-Rare Diseases
Some countries designate a subset of rare diseases to be ultra-rare. The UK has adopted an informal threshold of <1 per 50,000 people. Italy’s threshold is 1 case per 1,000,000 people. The US does not have a statutory definition for this category of diseases, but Congress is considering whether to establish a separate designation for ultra-rare diseases that would provide further incentive to develop orphan drugs targeting these diseases and improve patient access to treatment. For example, the proposed Speeding Therapy Access Today Act of 2021 included a provision to establish the Accelerating Lifesaving Therapies in Treating Ultra-rare Disease Entities program. If enacted, the program would recommend policies to address challenges associated with developing medical products for rare diseases or conditions in an individual or very small population. Manufacturers of drugs for rare diseases should be aware of similar proposals and consider the tradeoffs of establishing differentiated policy approaches for rare and ultra-rare diseases.
How Avalere Can Help
Avalere’s subject matter expertise within the rare disease landscape lends in-depth perspectives to support a variety of stakeholders interested in treatments for rare diseases. For more information on how Avalere can support your goals, connect with us.







