Variation in Generic Substitution Rates Among Part D Plans
Summary
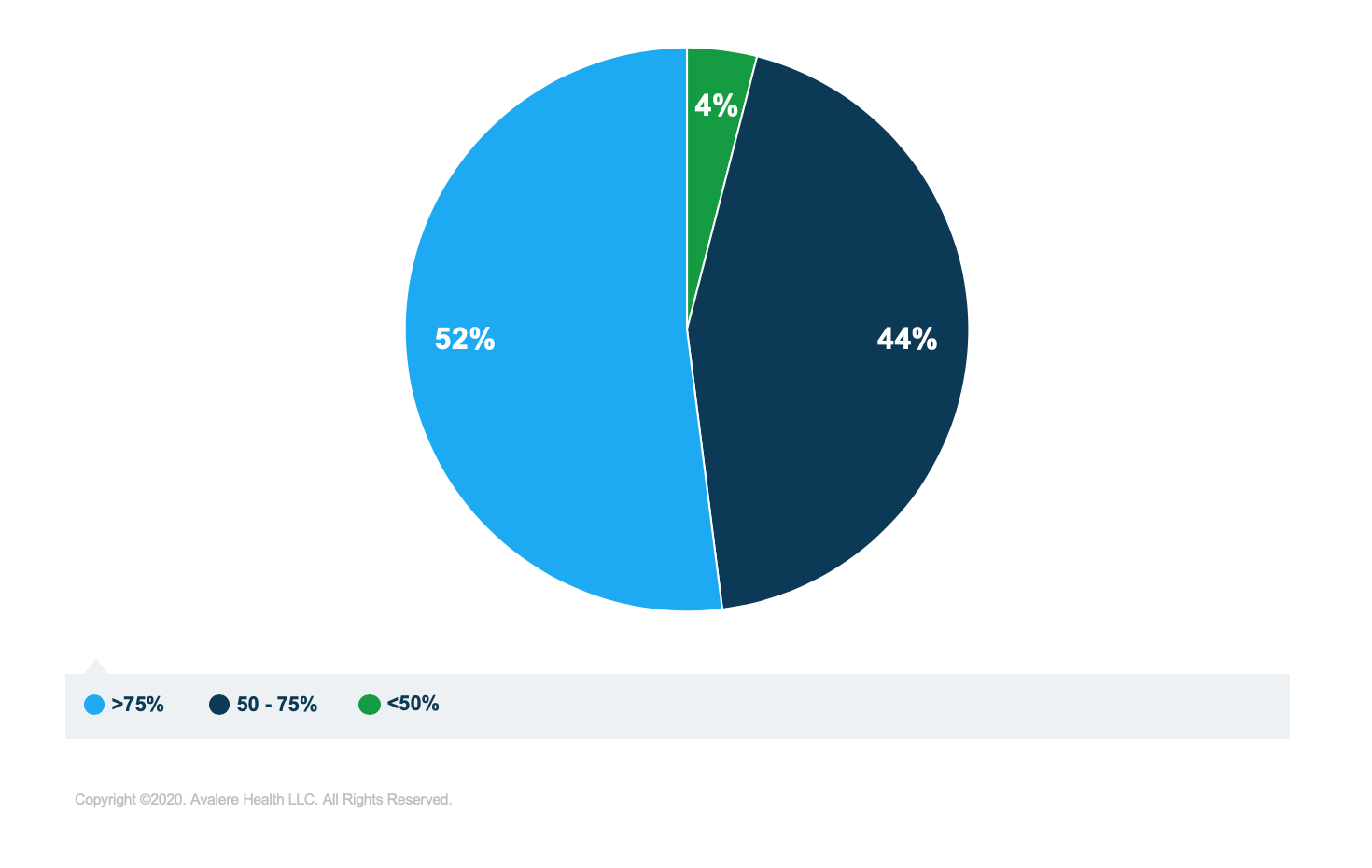
New analysis from Avalere finds 52% of Part D plans achieve generic substitution1 rates above 75%.Formulary guidelines in the Medicare Part D program have historically allowed Part D plans to manage prescription drug benefits to keep premiums low and drive utilization towards less expensive generic equivalents of brand drugs, resulting in patient and federal government savings. However, the recent Centers for Medicare & Medicaid Services (CMS) 2020 Annual Notice and Call Letter noted that there are instances where beneficiaries in select Part D plans are “not achieving optimal generic substitution.”
To evaluate this statement from CMS and to assess the rate at which generic drugs are filled in place of their brand equivalents, Avalere used Medicare Part D Prescription Drug Event (PDE) data to examine generic substitution rates by Part D plans.
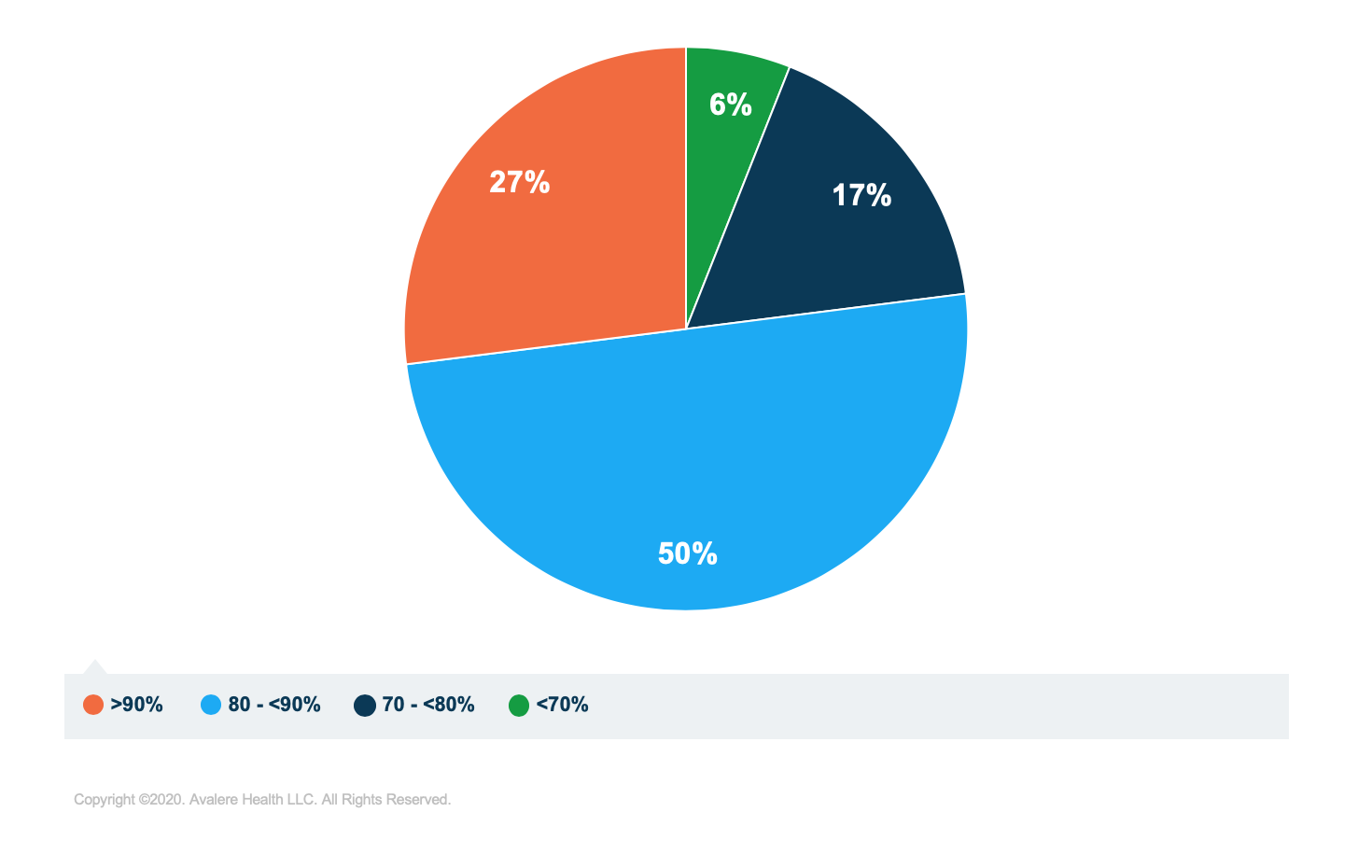
The analysis found variation in the rate at which generic substitution occurs among plans (Figure 1). Nearly half of plans had substitution rates below 75% in 2017, while 4% had substitution rates below 50%. When weighted by enrollment, the analysis found that 77% of beneficiaries are enrolled in plans with generic substitution rates above 80% (Figure 2), suggesting that most Part D beneficiaries are enrolled in plans that encourage generic substitution.
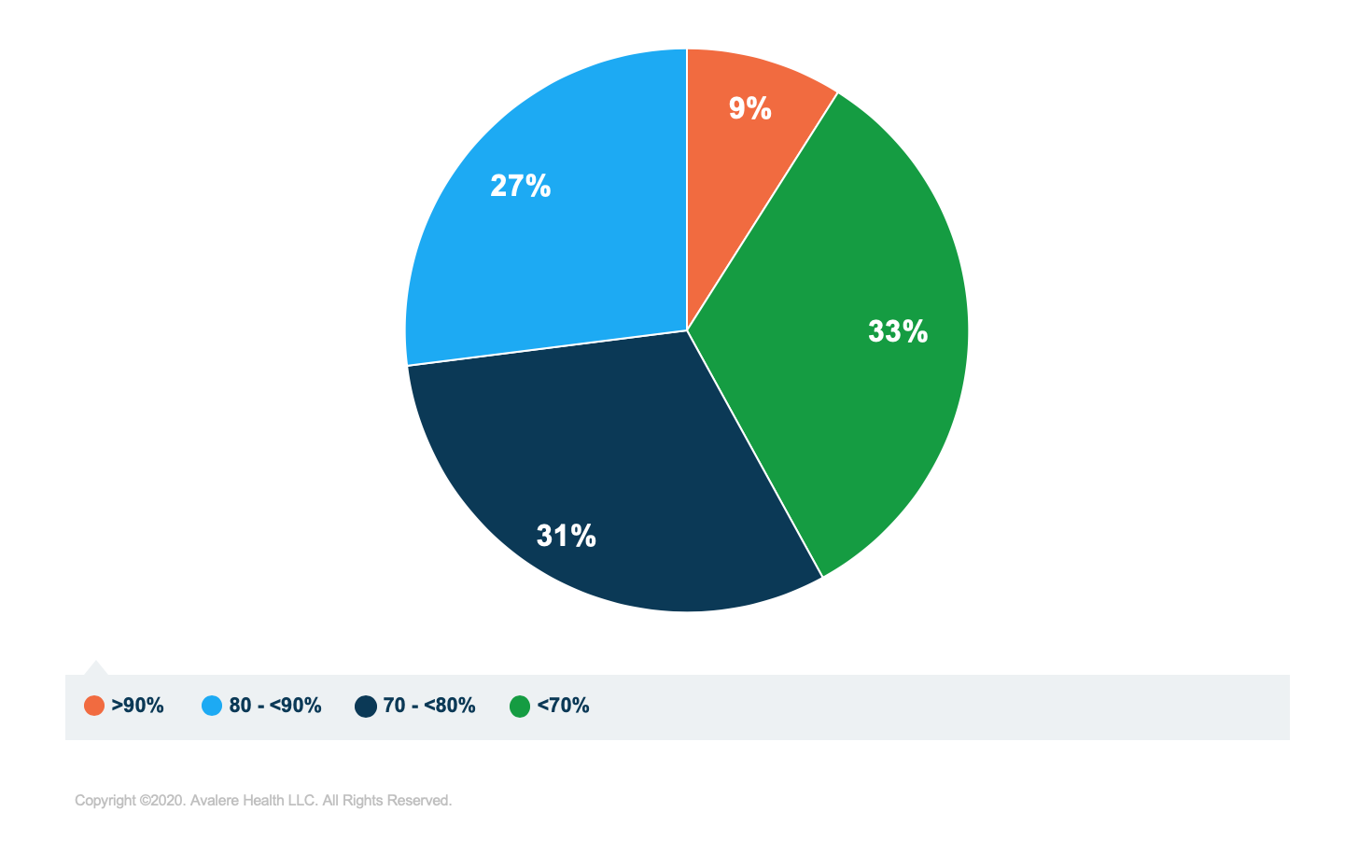
In addition, the analysis found that first-to-market generics2 launched in 2016 are substituted for their brand counterparts at lower rates than other generic products (Figure 3).
Avalere’s analysis also found variation in substitution rates among generic products by the price of their brand equivalent (Figure 4). While brand drugs with a Part D negotiated price of less than $450 were substituted for a generic equivalent at an average rate of 81%, this rate decreased as price increased. For brand products over $670, the threshold for the Medicare Part D specialty tier in 2019, substitution occurred only 69% of the time.
| Cost of the Brand 3 | Average Substitution Rate in Medicare Part D, 2017 |
|---|---|
| < $450 | 81% |
| $450+ | 71% |
| $500+ | 70% |
| $550+ | 70% |
| $600+ | 69% |
| $670+ | 69% |
Funding for this research was provided by the Association for Accessible Medicines. Avalere Health retained full editorial control.
To learn more about Avalere’s work in this space, connect with us.
Find out the top 2020 healthcare trends to watch.
Methodology
Avalere analyzed Medicare Part D Prescription Drug Event data from 2017, under the terms of a CMS research data use agreement (DUA), to determine the percentage of generic substitution by plan for the 2017 plan year. Per the DUA’s requirement, Avalere identified a random sample of 8.4M beneficiaries representing less than 20% of the total Part D population.
Avalere determined the generic substitution for a chemical entity by calculating the percentage of generic scripts as a percentage of all scripts for each chemical entity (both brand and generic). Avalere then calculated a weighted average number of brand and generic scripts for all Part D plans across all the chemical entities being filled through each plan. To determine the generic substitution rate for each Part D plan, Avalere calculated the percentage of weighted average number of generic scripts as a percentage of weighted average number of total scripts (both brand and generic) for that plan.
Avalere used the CMS’ Part D 30-day supply Negotiated Price file to identify the cost of the chemical entities at the National Drug Code level. Avalere then calculated the average substitution rates based on the different cost categories of interest.
For all the chemical entities included in the generic substitution analysis, Avalere used the Medispan® database to ensure that at least 1 brand drug and at least 1 generic drug are available on the market. For the first-to-market generic analysis, Avalere identified first-to-market generics that were approved by the Food and Drug Administration in 2016.
Notes
1. Generic substitution is often used to refer to the pharmacist-initiated substitution of a generic product dispensed in place of a brand product prescribed by a physician. However, in this context and for purpose of this analysis, generic substitution for a chemical entity is defined as the number of generic scripts as a percentage of all scripts for that chemical entity (both brand and generic).
2. First-to-market generics are the first generic product to be approved for a brand equivalent.
3. For the purposes of this analysis, brand cost is based on CMS’ Part D 30-day supply Negotiated Price file
Learn More



